Abstract
Introduction and hypothesis
The aim of this study was to evaluate a link between mesh infection and shrinkage.
Methods
Twenty-eight Wistar rats were implanted with synthetic meshes that were either non-absorbable (polypropylene (PP), n = 14) or absorbable (poly (d,l-lactic acid) (PLA94), n = 14). A validated animal incisionnal abdominal hernia model of mesh infection was used. Fourteen meshes (n = 7 PLA94 and n = 7 PP meshes) were infected intraoperatively with 10e6 CFU Escherichia coli, and compared with 14 non-infected meshes (n = 7 PLA94 and n = 7 PP meshes) (control groups). Explantations were performed on day 30. Shrinkage was evaluated by a reproducible numerical analysis of mesh area. Infection and histological study were evaluated on day 30.
Results
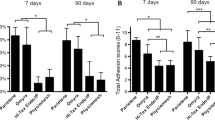
Non-infected meshes were less shrunk than infected meshes for both non-absorbable (5.0 ± 1.7% versus 21.6 ± 6.1%, p < 0.05) and absorbable meshes (2.4 ± 0.9% versus 11.0 ± 2.5%, p < 0.05).
Conclusion
This study highlights a link between infection and shrinkage in the model used.


Similar content being viewed by others
References
Collinet P, Belot F, Debodinance P, Ha Duc E, Lucot JP, Cosson M (2006) Transvaginal mesh technique for pelvic organ prolapse repair: mesh exposure management and risk factors. Int Urogynecol J Pelvic Floor Dysfunct 17:315–320
De Ridder D (2008) Should we use meshes in the management of vaginal prolapse? Curr Opin Urol 18:377–382
Bellon JM, Bujan J, Contreras L, Hernando A (1995) Integration of biomaterials implanted into abdominal wall: process of scar formation and macrophage response. Biomaterials 16:381–387
Birch C, Fynes MM (2002) The role of synthetic and biological prostheses in reconstructive pelvic floor surgery. Curr Opin Obstet Gynecol 14:527–535
Cosson M (2004) Risk of infection and prostheses: time out or a red flag? J Gynécol Obstét Biol Reprod 33:559–560
Dougherty SH (1988) Pathobiology of infection in prosthetic devices. Rev Infect Dis 10:1102–1117
An YH, Friedman RJ (1998) Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 43:338–348
Marshall K (1985) Mechanisms of bacterial adhesions at solid-water interfaces. In: Savage D, Fletcher M (eds) Bacterial adhesion. Mechanisms and physiological significance. Plenum, New York
Huang KH, Kung FT, Liang HM, Chang SY (2005) Management of polypropylene mesh erosion after intravaginal midurethral sling operation for female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 16:437–440
de Tayrac R, Oliva-Lauraire MC, Guiraud I, Henry L, Vert M, Mares P (2007) Long-lasting bioresorbable poly(lactic acid) 94 mesh: a new approach for soft tissue reinforcement based on an experimental pilot study. Int Urogynecol J Pelvic Floor Dysfunct 18:1007–1014
de Tayrac R, Chentouf S, Garreau H, Braud C, Guiraud I, Boudeville P, Vert M (2008) In vitro degradation and in vivo biocompatibility of poly(lactic acid) 94 mesh for soft tissue reinforcement in vaginal surgery. J Biomed Mater Res B Appl Biomater 85:529–536
Alponat A, Lakshminarasappa SR, Yavuz N, Goh PMY (1997) Prevention of adhesions by Seprefilm, an absorbable adhesion barrier: an incisional hernia model in rats. Am Surg 63:818–819
Zheng F, Lin Y, Verbeken E et al (2004) Host response after reconstruction of abdominal wall defects with porcine dermal collagen in a rat model. Am J Obstet Gynecol 191:1961–1970
Mathé ML, Lavigne JP, Oliva-Lauraire MC, Guiraud I, Marès P, de Tayrac R (2007) Comparison of different biomaterials for vaginal surgery using an in vivo model of meshes infection in rats. Gynécol Obstét Fertil 35:398–405
Hiltunen R, Nieminen K, Takala T et al (2007) Low weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 110:455–462
Nguyen JN, Burchette RJ (2008) Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 111:891–898
Sivaslioglu AA, Unlubilgin E, Dolen I (2008) A randomized comparison of polypropylene mesh surgery with site specific surgery in the treatment of cystocele. Int Urogynecol J Pelvic Floor Dysfunct 19:467–471
Weyhe D, Hoffmann P, Belyaev O, Mros K, Muller C, Uhl W, Schmitz F (2007) The role of TGF-beta1 as a determinant of foreign body reaction to alloplastic materials in rat fibroblast cultures: comparison of different commercially available polypropylene meshes for hernia repair. Regul Pept 138(1):10–14, Epub 2006 Sep 12
Sergent F, Desilles N, Lacoume Y, Bunel C, Marie JP, Marpeau L (2009) Experimental biomechanical evaluation of polypropylene prostheses used in pelvic organ prolapse surgery. Int Urogynecol J 20:597–604
Judlin P (2002) Infections in gynecology. Masson, Paris, pp p6–p7
Gristina AG (1987) Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237:1588–1595
Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V (2002) Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res 63:765–771
Conflicts of interest
This study represents a master thesis funded by Covidien. Renaud de Tayrac is a consultant for Covidien. The other co-authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mamy, L., Letouzey, V., Lavigne, JP. et al. Correlation between shrinkage and infection of implanted synthetic meshes using an animal model of mesh infection. Int Urogynecol J 22, 47–52 (2011). https://doi.org/10.1007/s00192-010-1245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-010-1245-7