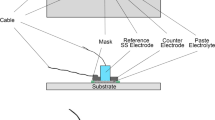
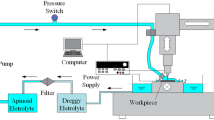
Abstract
To investigate the passivation behavior of stainless steels with various metallographic phases and their impact on micro-electrochemical machining (micro-ECM) performance, the electrochemical behavior of representative dual-phase stainless steel (SUS329J1), martensite stainless steel (SUS440C), ferritic stainless steel (SUS430), and austenite stainless steel (SUS316L) in NaClO3 and NaNO3 solutions were examined using potentiodynamic measurements and electrochemical impedance spectroscopy (EIS). EIS results indicate the charge-transfer resistance of the ferritic and martensite phases in the NaClO3 is higher than that in NaNO3, suggesting a stable passive film formation in the NaClO3 solution. Conversely, for the austenite phase, the passive film formed in NaNO3 is more stable than that formed in NaClO3. Experimental results from ECM reveal a different trend in material removal rates (MRRs) with the use of NaNO3 electrolyte SUS440C > SUS430 > SUS316L, contrasting with their respective MRR trends observed using NaClO3. Additionally, dual-phase steel SUS329J1 demonstrates a higher MRR than both SUS430 and SUS316L stainless steel. This study investigates the relationships between MRRs and metallographic phases of stainless steels, providing practical insights for optimizing electrolyte composition when machining different stainless steels.












Similar content being viewed by others

Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Liu GD, Karim MR, Arshad MH, Saxena KK, Liang W, Tong H, Li Y, Yang YX, Li CJ, Reynaerts D (2023) Tooling aspects of micro electrochemical machining (ECM) technology: design, functionality, and fabrication routes. J Mater Process Tech 320:118098. https://doi.org/10.1016/j.jmatprotec.2023.118098
Luo YG, Tong H, Liu GD, Wu TY, Li Y (2021) SACE scanning process assisted with ultrasonic vibration on quartz glass. Mater Manuf Process 37:1474–1482. https://doi.org/10.1080/10426914.2021.2006224
Byun JW, Shin HS, Kwon MH, Kim BH, Chu CN (2010) Surface texturing by micro ECM for friction reduction. Int J Precis Eng Manuf 11:747–753. https://doi.org/10.1007/s12541-010-0088-y
Liu GD, Li Y, Kong QC, Tong H (2016) Selection and optimization of electrolyte for micro electrochemical machining on stainless steel 304. Procedia CIRP 42:412–417. https://doi.org/10.1016/j.procir.2016.02.223
Duan S, Liu J, Wang J, Tang B, Zhu D (2023) Anodic electrochemical behaviors of Inconel 718 and Rene 65 alloys in NaNO3 solution. J Mater Process Tech 26:7312–7328. https://doi.org/10.1016/j.jmrt.2023.09.014
Wang D, Zhu Z, He B, Ge Y, Zhu D (2017) Effect of the breakdown time of a passive film on the electrochemical machining of rotating cylindrical electrode in NaNO3 solution. J Mater Process Tech 239:251–257. https://doi.org/10.1016/j.jmatprotec.2016.08.023
Luo H, Dong CF, Li XG, Xiao K (2012) The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different pH in the presence of chloride. Electrochim Acta 64:211–220. https://doi.org/10.1016/j.electacta.2012.01.025
Lee JS, Fushimi K, Nakanishi T, Hasegawa Y, Park YS (2014) Corrosion behaviour of ferrite and austenite phases on super duplex stainless steel in a modified green-death solution. Corros Sci 89:111–117. https://doi.org/10.1016/j.corsci.2014.08.014
Betova I, Bojinov M, Kinnunen P, Pohjanne P, Saario T (2002) Influence of the electrolyte composition and temperature on the transpassive dissolution of austenitic stainless steels in simulated bleaching solutions. Electrochim acta 47:3335–3349
Fattah-alhosseini A, Vafaeian S (2015) Passivation behavior of a ferritic stainless steel in concentrated alkaline solutions. J Mater Res Technol 4:423–428. https://doi.org/10.1016/j.jmrt.2015.02.003
Collazo A, Figueroa R, Mariño-Martínez C, Nóvoa XR, Pérez C (2023) Electrochemical characterization of a Fe-based shape memory alloy in an alkaline medium and the behaviour in aggressive conditions. Electrochim Acta 444:142034–142045. https://doi.org/10.1016/j.electacta.2023.142034
Liu GD, Tong H, Li Y, Tan QF, Zhu YL (2021) Passivation behavior of S136H steel in neutral electrolytes composed of NaClO3 and NaNO3 and its influence on micro electrochemical machining performance. Mater Today Commun 29:102762–102773. https://doi.org/10.1016/j.mtcomm.2021.102762
Deconinck D, Van Damme S, Deconinck J (2012) A temperature dependent multi-ion model for time accurate numerical simulation of the electrochemical machining process. Part I: Theoretical basis. Electrochim Acta 60:321–328. https://doi.org/10.1016/j.electacta.2011.11.070
Lohrengel MM, Rataj KP, Münninghoff T (2016) Electrochemical machining-mechanisms of anodic dissolution. Electrochim Acta 201:348–353. https://doi.org/10.1016/j.electacta.2015.12.219
Kong QC, Li Y, Liu GD, Li CJ, Tong H, Gan WM (2017) Electrochemical machining for micro holes with high aspect ratio on metal alloys using three-electrode PPS in neutral salt solution. Int J Adv Manuf Technol 93:1903–1913. https://doi.org/10.1007/s00170-017-0645-y
Liu GD, Tong H, Li Y, Zhong H (2020) Investigation on passivation and transient electrochemical behavior of Fe–Cr–Ni based alloy in micro ECM. J Electrochem Soc 167:063503. https://doi.org/10.1149/1945-7111/ab819a
Funding
This work was supported by the National Natural Science Foundation of China (grant number 52205439, 52375402), Beijing Natural Science Foundation (grant number L223016), and China Space Foundation Aerospace Propulsion Public Welfare Special Fund (grant number KDJJ20230502014).
Author information
Authors and Affiliations
Contributions
Guodong Liu: conceptualization, formal analysis, data curation, validation, roles/writing—original draft. Jingyao Shi: original idea, funding acquisition, methodology, writing—reviewing and editing. Yuxin Yang: investigation. Zeyu Gong: investigation. Chaojiang Li: supervision, resources, project administration.
Corresponding author
Ethics declarations
Ethical approval
There are no ethical issues to declare.
Consent to participate
All the authors participate in the research work of this paper.
Consent to publish
All authors agree to publish the research results in this paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Shi, J., Yang, Y. et al. Effects of passivation behavior on micro-electrochemical machining (ECM) performance of stainless steels with different metallographic phases in NaNO3 and NaClO3 solutions. Int J Adv Manuf Technol 130, 3867–3876 (2024). https://doi.org/10.1007/s00170-023-12934-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-023-12934-5