Abstract
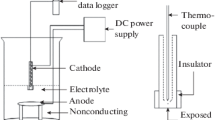
For many applications, aluminum alloys require anodization to produce alumina films that protect the surfaces against corrosion and abrasive wear. This process is traditionally carried out by dipping the substrate into a suitable bath of electrolyte. A study on the effectiveness of a direct writing pen for localized anodization of aluminum 6061 is presented. The writing pen was designed for applications in which complete submersion of the substrate in an electrolyte is inconvenient or unnecessary. Three aqueous electrolyte solutions were used: 15% sulfuric acid; 10% sulfuric acid plus 5% citric acid; and 5% sulfuric acid plus 10% citric acid. All concentrations are in v/v%. Voltage levels of 30, 40, and 50 V were applied, while the writing traverse speeds were 0.1, 1, and 3 \(\mathrm{mm}/\mathrm{s}\) on different experimental samples. Single-step and two-step anodization were implemented. The anodic oxide films were characterized for surface morphology, thickness, micro-hardness, and ability to hold dyes through the nanopores. The results show that the lower the pen traverse speeds, the higher the thickness of the alumina films produced. The thicknesses obtained were highest at 21.5 µm for oxide films generated at 0.1 \(\mathrm{mm}/\mathrm{s}\) traverse speed and 50 \(\mathrm{V}\) using sulfuric acid in a single-step anodization. However, the hardest surfaces were obtained for samples processed at 50 \(\mathrm{V}\) with two-step anodization and 1 \(\mathrm{mm}/\mathrm{s}\) traverse speed, yielding 596 \(\mathrm{Hv}.\) The direct writing pen was found to demonstrate effectiveness for localized surface anodization with minimal environmental impact and it would be cost-effective in niche applications.















Similar content being viewed by others
References
Yao C, Perla V, McKenzie JL, Slamovich EB, Webster TJ (2005) Anodized Ti and Ti6Al4V possessing nanometer surface features enhances osteoblast adhesion. J Biomed Nanotechnol 1(1):68–73
Williamson RS, Disegi J, Janorkar AV, Griggs JA, Roach MD (2015) Effect of duty cycle on the crystallinity, pore size, surface roughness and corrosion resistance of the anodized surface on titanium. Surf Coat Technol 277:278–288
Zaraska L, Gawlak K, Gurgul M, Gilek D, Kozieł M, Socha RP, Sulka GD (2019) Morphology of nanoporous anodic films formed on tin during anodic oxidation in less commonly used acidic and alkaline electrolytes. Surf Coat Technol 362:191–199
Allam NK, Grimes CA (2011) Electrochemical fabrication of complex copper oxide nanoarchitectures via copper anodization in aqueous and non-aqueous electrolytes. Mater Lett 65(12):1949–1955
Pauric AD, Baig SA, Pantaleo AN, Wang Y, Kruse P (2012) Sponge-like porous metal surfaces from anodization in very concentrated acids. J Electrochem Soc 160(1):C12
Salman SA, Okido M (2013) Anodization of magnesium (Mg) alloys to improve corrosion resistance. Corrosion prevention of magnesium alloys. Woodhead Publishing Series in Metals and Surface Engineering, Elsevier, pp 197–231
Simchen F, Sieber M, Kopp A, Lampke T (2020) Introduction to plasma electrolytic oxidation—an overview of the process and applications. Coatings 10(7):628
Cho YS, Liao LK, Hsu CH, Hsu YH, Wu WY, Liao SC,...& Lien SY (2019). Effect of substrate bias on biocompatibility of amorphous carbon coatings deposited on Ti6Al4V by PECVD. Surf Coat Technol, 357, 212–217.
Bai Y, Xi Y, Gao K, Yang H, Pang X, Yang X, Volinsky AA (2019) Brittle coating effects on fatigue cracks behavior in Ti alloys. Int J Fatigue 125:432–439
Zhu L, Xue P, Lan Q, Meng G, Ren Y, Yang Z,...& Liu Z (2021) Recent research and development status of laser cladding: a review. Optics & laser technology, 138, 106915
Aykol M, Persson KA (2018) Oxidation protection with amorphous surface oxides: thermodynamic insights from ab initio simulations on aluminum. ACS Appl Mater Interfaces 10(3):3039–3045
Evertsson J, Bertram F, Zhang F, Rullik L, Merte LR, Shipilin M,...& Lundgren E (2015) The thickness of native oxides on aluminum alloys and single crystals. Applied surface science, 349, 826–832.
Guo F, Cao Y, Wang K, Zhang P, Cui Y, Hu Z, Xie Z (2022) Effect of the anodizing temperature on microstructure and tribological properties of 6061 aluminum alloy anodic oxide films. Coatings 12(3):314
Cabral-Miramontes J, Gaona-Tiburcio C, Estupinán-López F, Lara-Banda M, Zambrano-Robledo P, Nieves-Mendoza D,...& Almeraya-Calderón F (2020) Corrosion resistance of hard coat anodized AA 6061 in citric–sulfuric solutions. Coatings, 10(6), 601.
Li J, Wei H, Zhao K, Wang M, Chen D, Chen M (2020) Effect of anodizing temperature and organic acid addition on the structure and corrosion resistance of anodic aluminum oxide films. Thin Solid Films 713:138359
Cabral-Miramontes J, Almeraya-Calderón F, López FE, Lara Banda M, Olguín-Coca J, López-León LD,...& Gaona-Tiburcio C (2021) Citric acid as an alternative to sulfuric acid for the hard-anodizing of AA6061. Metals, 11(11), 1838.
Chu SZ, Wada K, Inoue S, Isogai M, Katsuta Y, Yasumori A (2006) Large-scale fabrication of ordered nanoporous alumina films with arbitrary pore intervals by critical-potential anodization. J Electrochem Soc 153(9):B384
Lee W, Park SJ (2014) Porous anodic aluminum oxide: anodization and templated synthesis of functional nanostructures. Chem Rev 114(15):7487–7556
Paz Martínez-Viademonte M, Abrahami ST, Hack T, Burchardt M, Terryn H (2020) A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 10(11):1106
O’sullivan JP, Wood GC (1970) The morphology and mechanism of formation of porous anodic films on aluminium. Proceedings of the Royal Society of London. Math Phys Sci 317(1531):511–543
Garcia-Vergara SJ, Skeldon P, Thompson GE, Hashimoto T, Habazaki H (2007) Compositional evidence for flow in anodic films on aluminum under high electric fields. J Electrochem Soc 154(9):C540
Garcia-Vergara SJ, Skeldon P, Thompson GE, Habazaki H (2007) Stress generated porosity in anodic alumina formed in sulphuric acid electrolyte. Corros Sci 49(10):3772–3782
Skeldon P, Thompson GE, Garcia-Vergara SJ, Iglesias-Rubianes L, Blanco-Pinzon CE (2006) A tracer study of porous anodic alumina. Electrochem Solid-State Lett 9(11):B47
MIL-A-8625F (1993) Anodic Coatings for Aluminum and Aluminum Alloys. Departments and Agencies ofthe Department of Defense: the Pentagon Arlington, VA, USA, pp 1–19
Kim KM, Lee YK, Shin SH, Choi SM, Kwon YS (2008) Aluminum packaging for light-emitting diode using selectively anodizing method. Jpn J Appl Phys 47(4S):2950
Yeo SK, Chun JH, Kim KM, Yook JM, Kwon YS (2007) An X-band high power amplifier module package using selectively anodized aluminum substrate. Proceedings of the 37th European Microwave Conference. IEEE, pp 1357–1360
Yook JM, Yu JI, Kim YJ, Kwon YS (2009) Integrated passive devices on the selectively anodized aluminum oxide. Proceedings of European Microwave Conference (EuMC). IEEE, pp 1654–1657
Park J, Fattaccioli J, Fujita H, Kim B (2012) Fabrication of aluminum/alumina patterns using localized anodization of aluminum. Int J Precis Eng Manuf 13:765–770
Vanek DL (2002) Selective electrofinishing. Met Finish 100:345–358
Norrls, J. C., & Platlng, S. S. brush and flow selective sulfuric acid anodizing. https://kipdf.com/i-they-are-portable_5b00ce7e8ead0e8e6c8b4595.html. Accessed on December 2, 2022.
Quitzke S, Danilov I, Martin A, Morgenstern R, Lampke T, Schubert A (2023) Simulation-assisted process design and experimental verification of laterally confined oxide areas generated with continuous electrolytic free jet on EN AW-7075 aluminum alloy. Micromachines 14(2):293
Kuhn D, Martin A, Eckart C, Sieber M, Morgenstern R, Hackert-Oschätzchen M, ... & Schubert A (2017) Localised anodic oxidation of aluminium material using a continuous electrolyte jet. In IOP conference series: materials science and engineering (Vol. 181, No. 1, p. 012042). IOP Publishing
Nakajima D, Kikuchi T, Natsui S, Suzuki RO (2014) Growth behavior of anodic oxide formed by aluminum anodizing in glutaric and its derivative acid electrolytes. Appl Surf Sci 321:364–370
Sulka GD, Stroobants S, Moshchalkov V, Borghs G, Celis JP (2002) Synthesis of well-ordered nanopores by anodizing aluminum foils in sulfuric acid. J Electrochem Soc 149(7):D97
Sulka GD, Parkoła KG (2006) Anodising potential influence on well-ordered nanostructures formed by anodisation of aluminium in sulphuric acid. Thin Solid Films 515(1):338–345
Kim HS, Kim DH, Lee W, Cho SJ, Hahn JH, Ahn HS (2010) Tribological properties of nanoporous anodic aluminum oxide film. Surf Coat Technol 205(5):1431–1437
Cheng TC, Chou CC (2015) The electrical and mechanical properties of porous anodic 6061–T6 aluminum alloy oxide film. J Nanomater 16(1):141–141
Peng L, Li M (2015) Improved technology for hard anodizing dissolution of aluminum alloy part. Open Mater Sci J 9(1):82–85
Kwolek P, Krupa K, Obłój A, Kocurek P, Wierzbińska M, Sieniawski J (2018) Tribological properties of the oxide coatings produced onto 6061–T6 aluminum alloy in the hard anodizing process. J Mater Eng Perform 27:3268–3275
Funding
This project was funded by WiSys Technology Foundation through the WiSys Prototyping Center. The views expressed herein are those of the authors and are not necessarily those of WiSys.
Author information
Authors and Affiliations
Contributions
JO conceived the design of the experiment and supervised its implementation. GS III contributed to the modification of the original pen design and data collections. MD contributed to the data collections. The first draft of the manuscript was written by JO and all authors made their comments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obielodan, J.O., Schwarzmann, G. & Delwiche, M. Effects of speeds, voltages, and electrolytes on localized anodization of Al 6061 alloys using a direct writing pen. Int J Adv Manuf Technol 130, 1653–1664 (2024). https://doi.org/10.1007/s00170-023-12782-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-023-12782-3