Abstract
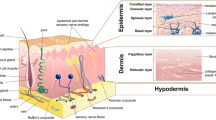
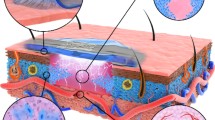
Tissue reconstruction through in vitro cell seeding is a popular method for tissue engineering. In this paper, we proposed a thin-layer structure consisting of multiple hexagons for the regeneration of skin tissue. Cells could be seeded and cultured within the structure via dielectrophoresis (DEP) actively. A thin layer of the structure was fabricated with biocompatible medical-grade stainless steel via precise laser cutting. The fabricated layers were stacked together to form a 3D electrode pair, which could be used to generate a 3D electric field. Thus, the suspended cells within the structure could be patterned via DEP manipulation. The input voltage was examined and optimized to ensure cell viability and patterning efficiency during the DEP manipulation process. As soon as we applied the optimized voltage, human foreskin fibroblast (HFF) cells could be attracted along the edge of the electrodes, forming hexagonal cellular patterns. After that, a single layer of the patterned cells was further cultured in an incubator for 7 days and observed under a microscope. The obtained images showed that the seeded cells could proliferate and fill in the hexagonal wells, which could be used for further skin tissue regeneration. As shown in the experimental results, this structure could be used for active cell seeding and proliferation for the development of skin tissue engineering.











Similar content being viewed by others
References
Lantada D, Andrés AI, Hernán, Pareja Sánchez B (2014) Free-form rapid prototyped porous pdms scaffolds incorporating growth factors promote chondrogenesis. Adv Mater Sci Eng 1:1–10
Vashisth P, Nikhil K, Roy P, Pruthi PA, Singh RP, Pruthi V (2016) A novel gellan-PVA nanofibrous scaffold for skin tissue regeneration: fabrication and characterization. Carbohydr Polym 136:851–859
Sadeghi-Avalshahr A, Nokhasteh S, Molavi AM, Khorsand-Ghayeni M, Mahdavi-Shahri M (2017) Synthesis and characterization of collagen/PLGA biodegradable skin scaffold fibers. Regen Biomater 4(5):309–314
Zhang X, Jia C, Qiao X, Liu T, Sun K (2016) Porous poly(glycerol sebacate) (PGS) elastomer scaffolds for skin tissue engineering. Polym Test 54:118–125
Oliveira, Pires Liliana Sofia , F. M. H. F. Vaz , and de Oliveira José Martinho Marques (2018) "Biofabrication of glass scaffolds by 3D printing for tissue engineering." Int J Adv Manuf Technol, 98, 2665–2676
Mihailescu M, Paun IA, Zamfirescu M, Luculescu CR, Acasandrei AM, Dinescu M (2016) Laser-assisted fabrication and non-invasive imaging of 3d cell-seeding constructs for bone tissue engineering. J Mater Sci 51(9):4262–4273
Xie M, Mills J, Wang Y, Mahmoodi M, Sun D (2016) Automated translational and rotational control of biological cells with a robot-aided optical tweezers manipulation system. IEEE Trans Autom Sci Eng 13(2):543–551
Xie M, Li X, Wang Y, Liu Y, Sun D (2017) Saturated PID control for the optical manipulation of biological cells. IEEE Trans Control Syst Technol 99:1–8
Xie M, Shakoor A, Shen Y, Mills JK, Sun D (2019) Out-of-plane rotation control of biological cells with a robot-tweezers manipulation system for orientation-based cell surgery. IEEE Trans Biomed Eng 66:199–207
Xie M, Shakoor A, Wu C (2018) Manipulation of biological cells using a robot-aided optical tweezers system. Micromachines 9(5):245
Xie M, Mills J, Li X, Wang Y, Sun D (2015) Modelling and Control of Optical Manipulation for Cell Rotation. IEEE International Conference on Robotics and Automation, 956–961.
Xu F, Finley TD, Turkaydin M, Sung Y, Gurkan UA, Yavuz AS, Guldiken RO, Demirci U (2011) The assembly of cell-encapsulating microscale hydrogels using acoustic waves. Biomaterials 32(31):7847–7855
Ding X, Lin SCS, Kiraly B, Yue H, Li S, Chiang IK, Shi J, Benkovic SJ, Huang TJ (2012) On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc Natl Acad Sci 109(28):11105–11109
Coquelle E, Bossis G (2006) Mullins effect in elastomers filled with particles aligned by a magnetic field. Int J Solids Struct 43:7659–7672
Baghani M, Mohammadi M, Farajpour A (2016) Dynamic and stability analysis of the rotating nanobeam in a non-uniform magnetic field considering the surface energy. Int J Appl Mech 08(04):1650048
Ma W, Li J, Niu F, Ji H, Sun D (2017) Robust control to manipulate a microparticle with electromagnetic coil system. IEEE Trans Ind Electron 64(11):8566–8577
Alazzam A, Stiharu I, Bhat R, Meguerditchian AN (2011) Interdigitated comb-like electrodes for continuous separation of malignant cells from blood using dielectrophoresis. Electrophoresis 32(11):1327–1336
Ho CT, Lin RZ, Chen RJ, Chin CK, Gong SE, Chang HY, Peng HL, Hsu L, Yew TR, Chang SF, Liu CH (2013) Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. Lab Chip 13(18):3578–3587
Pohl HA (1978) Dielectrophoresis. Cambridge University Press, Cambridge
Hughes MP (2002) Strategies for dielectrophoretic separation in laboratory-on-a-chip systems. Electrophoresis 23(16):2569–2582
Pethig R, Huang Y (1995) Dielectrophoretic and electrorotation behaviour of cells: theory and experiment. Bioelectrochemistry of Cells and Tissues, Birkhäuser Basel
Dewidar, M. M., Khalil, K. A. and Lim, J. K. (2007) “Processing and mechanical properties of porous 316l stainless steel for biomedical applications,” Trans Nonferrous Metals Soc China, 17(3), 0–473, 468
Hu J, Zhou Y, and Liu Z (2018) “The friction effect on buckling behavior of cellular structures under axial load,” International Journal of Applied Mechanics, S1758825118500138
Zargar R, Nourmohammadi J, Amoabediny G (2016) Preparation, characterization, and silanization of 3d microporous PDMS structure with properly sized pores for endothelial cell culture. Biotechnol Appl Biochem 63(2):190–199
Niepel MS, Almouhanna F, Ekambaram BK, Menzel M, Heilmann A and Groth T (2018) “Cross-linking multilayers of poly-\r, l\r, −lysine and hyaluronic acid: effect on mesenchymal stem cell behavior,” Int J Artif Organs, 039139881775259
Yoshioka T, Kunimori R, Hisaoka I, Nagasawa H, Kanezashi M, Tsuru T (2019) Molecular dynamics simulation study on the mechanisms of liquid-phase permeation in nanopores. Sep Purif Technol 220:259–267. https://doi.org/10.1016/j.seppur.2019.03.014
Chu HK, Huan Z, Mills JK, Yang J, Sun D (2015) Three-dimensional cell manipulation and patterning using dielectrophoresis via a multi-layer scaffold structure. Lab Chip 15(3):920–930
Huan Z, Chu HK, Yang J, Sun D (2017) Characterization of a Honeycomb-like Scaffold with Dielectrophoresis-based Patterning for Tissue Engineering. IEEE trans Biomed Eng 64(4):755–764
Huan Z, Chu HK, Liu H, Yang J, Sun D (2017) Engineered bone scaffold with dielectrophoresis-based patterning using 3D printing. Biomed Microdevices 19:102
Huang C, Liu C, Loo J, Stakenborg T, Lagae L (2014) Single cell viability observation in cell dielectrophoretic trapping on a microchip. Appl Phys Lett 104(1):2216
Zhitomirsky B, Farber H, Assaraf YG (2018) Lysotracker and mitotracker red are transport substrates of p-glycoprotein: implications for anticancer drug design evading multidrug resistance. J Cell Mol Med 22(4):2131–2141
Funding
This work was supported in part by the Natural Science Foundation of Fujian Province (No. 2019J01869 and No. 2019J05124), the Education Research Project for Young and Middle-aged Teachers of Fujian Province (No. JT180425 and No. JT180426), the Scientific Research Foundation for High Level Talents of Xiamen University of Technology (No. YKJ17012R and No. YKJ17013R), and the Promotion Scientific Research Project Young Teachers (No. XPDKQ18018 and No. XPDKQ18017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huan, Z., Ma, W., Xu, M. et al. Cell patterning via optimized dielectrophoretic force within hexagonal electrodes in vitro for skin tissue engineering. Int J Adv Manuf Technol 105, 4899–4907 (2019). https://doi.org/10.1007/s00170-019-04072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-019-04072-8