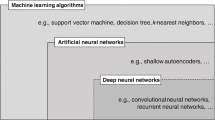
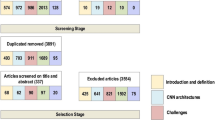
Abstract
Deep learning has the potential to be one of the most transformative technologies to impact orthopedic surgery. Substantial innovation in this area has occurred over the past 5 years, but clinically meaningful advancements remain limited by a disconnect between clinical and technical experts. That is, it is likely that few orthopedic surgeons possess both the clinical knowledge necessary to identify orthopedic problems, and the technical knowledge needed to implement deep learning-based solutions. To maximize the utilization of rapidly advancing technologies derived from deep learning models, orthopedic surgeons should understand the steps needed to design, organize, implement, and evaluate a deep learning project and its workflow. Equipping surgeons with this knowledge is the objective of this three-part editorial review. Part I described the processes involved in defining the problem, team building, data acquisition, curation, labeling, and establishing the ground truth. Building on that, this review (Part II) provides guidance on pre-processing and augmenting the data, making use of open-source libraries/toolkits, and selecting the required hardware to implement the pipeline. Special considerations regarding model training and evaluation unique to deep learning models relative to “shallow” machine learning models are also reviewed. Finally, guidance pertaining to the clinical deployment of deep learning models in the real world is provided. As in Part I, the focus is on applications of deep learning for computer vision and imaging.







Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Ahn G, Choi BS, Ko S, Jo C, Han HS, Lee MC et al (2022) High-resolution knee plain radiography image synthesis using style generative adversarial network adaptive discriminator augmentation. J Orthop Res. https://doi.org/10.1002/jor.25325
Anaconda. https://www.anaconda.com. Accessed 29 Oct 2022
Bernstein D (2014) Containers and cloud: from LXC to docker to kubernetes. IEEE Cloud Computing 1:81–84
Chung SW, Han SS, Lee JW, Oh KS, Kim NR, Yoon JP et al (2018) Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop 89:468–473
fast.ai. https://docs.fast.ai. Accessed 29 Oct 2022
Frid-Adar M, Diamant I, Klang E, Amitai M, Goldberger J, Greenspan H (2018) GAN-based synthetic medical image augmentation for increased CNN performance in liver lesion classification. Neurocomputing 321:321–331
Galbusera F, Bassani T, Casaroli G, Gitto S, Zanchetta E, Costa F et al (2018) Generative models: an upcoming innovation in musculoskeletal radiology? A preliminary test in spine imaging. Eur Radiol Exp 2:29
GitHub. https://github.com. Accessed 31 Oct 2022
Goodfellow I, Bengio Y, Courville A (2016) Deep learning. MIT Press
Hightower K, Burns B, Beda J (2017) Kubernetes: up and running dive into the future of infrastructure. O’Reilly Media, Inc
Horng MH, Kuok CP, Fu MJ, Lin CJ, Sun YN (2019) Cobb angle measurement of spine from x-ray images using convolutional neural network. Comput Math Methods Med 2019:6357171
Jupyter. https://jupyter.org. Accessed 29 Oct 2022
Keras. https://keras.io. Accessed 29 Oct 2022
Li YC, Chen HH, Horng-Shing LH, Hondar Wu HT, Chang MC, Chou PH (2021) Can a deep-learning model for the automated detection of vertebral fractures approach the performance level of human subspecialists? Clin Orthop Relat Res 479:1598–1612
NVIDIA cuDNN. https://developer.nvidia.com/cudnn. Accessed 30 Oct 2022
Oeding JF, Williams RJ, Nwachukwu BU, Martin RK, Kelly BT, Karlsson J et al (2022) A practical guide to the development and deployment of deep learning models for the Orthopedic surgeon: part I. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-022-07239-1
Pruneski JA, Williams RJ, Nwachukwu BU, Ramkumar PN, Kiapour AM, Martin RK et al (2022) The development and deployment of machine learning models. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-022-07155-4
Python. https://www.python.org. Accessed 29 Oct 2022
PyTorch. https://pytorch.org. Accessed 29 Oct 2022
Shin H-C, Tenenholtz NA, Rogers JK, Schwarz CG, Senjem ML, Gunter JL et al (2018) Medical image synthesis for data augmentation and anonymization using generative adversarial networks. In: Gooya A, Goksel O, Oguz I, Burgos N (eds) Simulation and synthesis in medical imaging. Springer International Publishing, pp 1–11
TensorFlow. https://www.tensorflow.org. Accessed 29 Oct 2022
Thambawita V, Strümke I, Hicks SA, Halvorsen P, Parasa S, Riegler MA (2021) Impact of image resolution on deep learning performance in endoscopy image classification: an experimental study using a large dataset of endoscopic images. Diagnostics (Basel) 11(12):2183
Thian YL, Li Y, Jagmohan P, Sia D, Chan VEY, Tan RT (2019) Convolutional neural networks for automated fracture detection and localization on wrist radiographs. Radiol Artif Intell 1:e180001
Wolterink JM, Mukhopadhyay A, Leiner T, Vogl TJ, Bucher AM, Išgum I (2021) Generative adversarial networks: a primer for radiologists. Radiographics 41:840–857
Funding
No funding was needed for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oeding, J.F., Williams, R.J., Camp, C.L. et al. A practical guide to the development and deployment of deep learning models for the orthopedic surgeon: part II. Knee Surg Sports Traumatol Arthrosc 31, 1635–1643 (2023). https://doi.org/10.1007/s00167-023-07338-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-023-07338-7