Abstract
Purpose
Rotator cuff tendon–bone healing often leads to scarring and low biomechanical strength, resulting in a tendency to re-tear. This study examined whether combining autologous osteochondral transplantation and periosteum transplantation increases fibrocartilage transition zone regeneration and improves biomechanical fixation.
Methods
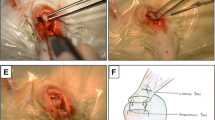
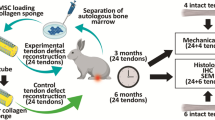
A total of 48 New Zealand white rabbits were divided into the periosteum, autologous osteochondral, combination of autologous osteochondral and periosteum, and control groups. The supraspinatus tendon was cut from the greater tuberosity and repaired by different transplants. A total of 12 rabbits were used for histological examination (haematoxylin and eosin staining, Masson’s staining and Safranin-O staining) at 4, 8 and 12 weeks after the repair, and 36 rabbits were used for biomechanical tests (maximal failure load and stiffness).
Results
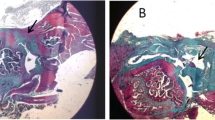
At 4 weeks following the operation, each group had a large tendon–bone gap with a small number of disordered collagen fibres. At 8 weeks, the tendon–bone gap was smaller than that before the operation, and the tendon–bone gap in each experimental group was smaller with neater and denser collagen fibres and chondrocytes than in the control group, with the osteochondral combined periosteum group having the best results. At 12 weeks, the typical tendon–bone transitional structure was observed in the osteochondral combined periosteum group, and more collagen fibres and chondrocytes were generated in each group. The osteochondral combined periosteum group had the largest staining area and the largest amount of cartilage. The maximum tensile strength and stiffness of each group increased over time. There was no significant difference in each group’s maximum tensile strength and stiffness at 4 weeks after the operation. However, the maximum tensile strength and stiffness of the osteochondral combined periosteum group at 8 and 12 weeks after operation were significantly higher than those of other groups (P < 0.05).
Conclusion
Histological and biomechanical results show that autologous osteochondral transplantation combined with periosteum transplantation can effectively promote the regeneration of fibrous cartilage in the tendon–bone junction of the rotator cuff. It is concluded that this technique is a new treatment method to promote tendon–bone healing in the rotator cuff.





Similar content being viewed by others
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RCT:
-
Rotator cuff tear
- ARCR:
-
Arthroscopic rotator cuff repair
- HE:
-
Haematoxylin and eosin
References
Bao D, Sun J, Gong M, Shi J, Qin B, Deng K et al (2021) Combination of graphene oxide and platelet-rich plasma improves tendon-bone healing in a rabbit model of supraspinatus tendon reconstruction. Regen Biomater 8(6):1–11
Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR (2002) The skeletal attachment of tendons–tendon “entheses.” Comp Biochem Physiol A Mol Integr Physiol 133:931–945
Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ (1998) Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg 7:599–605
Chang CH, Chen CH, Su CY, Liu HT, Yu CM (2009) Rotator cuff repair with periosteum for enhancing tendon-bone healing: a biomechanical and histological study in rabbits. Knee Surg Sports Traumatol Arthrosc 17:1447–1453
Chen BH (2014) Reliability of reagent strips for the measurement of glucosuria in a neonatal intensive care setting. Pediatr Neonatol 55:424–425
Chen Y, Ouyang X, Wu Y, Guo S, Xie Y, Wang G (2020) Co-culture and mechanical stimulation on mesenchymal stem cells and chondrocytes for cartilage tissue engineering. Curr Stem Cell Res Ther 15:54–60
Cheng B, Ge H, Zhou J, Zhang Q (2014) TSG-6 mediates the effect of tendon derived stem cells for rotator cuff healing. Eur Rev Med Pharmacol Sci 18:247–251
Chung SW, Oh JH, Gong HS, Kim JY, Kim SH (2011) Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med 39:2099–2107
Cui XM, Wu JQ, Xiong XT, Hua Q, Zuo Y, Shi HY (2022) Co-culture of bone marrow mesenchymal stem cells with articular cartilage block in rabbits. OJC 28(04):349–355
de Almeida Filho IA, Coelho DA (2021) Rotator cuff healing. Rev Bras Ortop (Sao Paulo) 56:291–298
Dey Hazra RO, Ernat JJ, Rakowski DR, Boykin RE, Millett PJ (2021) The evolution of arthroscopic rotator cuff repair. Orthop J Sports Med 9:23259671211050900
Han L, Fang WL, Jin B, Xu SC, Zheng X, Hu YG (2019) Enhancement of tendon-bone healing after rotator cuff injuries using combined therapy with mesenchymal stem cells and platelet rich plasma. Eur Rev Med Pharmacol Sci 23:9075–9084
Hsu MJ, Christ M, Christ B (2021) Co-culture of human mesenchymal stromal cells and primary mouse hepatocytes. Methods Mol Biol 2269:151–165
Huang K, Du J, Xu J, Wu C, Chen C, Chen S et al (2022) Tendon-bone junction healing by injectable bioactive thermo-sensitive hydrogel based on inspiration of tendon-derived stem cells. Mater Today Chem 23:100720
Killian ML, Cavinatto L, Shah SA, Sato EJ, Ward SR, Havlioglu N et al (2014) The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. J Orthop Res 32:439–447
Kovacevic D, Suriani RJ Jr, Grawe BM, Yian EH, Gilotra MN, Hasan SA et al (2020) Management of irreparable massive rotator cuff tears: a systematic review and meta-analysis of patient-reported outcomes, reoperation rates, and treatment response. J Shoulder Elbow Surg 29:2459–2475
Kwon J, Kim YH, Rhee SM, Kim TI, Lee J, Jeon S et al (2018) Effects of allogenic dermal fibroblasts on rotator cuff healing in a rabbit model of chronic tear. Am J Sports Med 46:1901–1908
Lebaschi AH, Deng XH, Camp CL, Zong J, Cong GT, Carballo CB et al (2018) Biomechanical, histologic, and molecular evaluation of tendon healing in a new murine model of rotator cuff repair. Arthroscopy 34:1173–1183
Leung KS, Qin L, Fu LK, Chan CW (2002) A comparative study of bone to bone repair and bone to tendon healing in patella-patellar tendon complex in rabbits. Clin Biomech (Bristol, Avon) 17:594–602
Liu JX, Gao YL, Zhang GR, Min SC, Dong HT, An LP et al (2019) Follow-up study on autogenous osteochondral transplantation for cartilage defect of knee joint. Zhongguo Gu Shang 32:346–349
Liu Q, Hatta T, Qi J, Liu H, Thoreson AR, Amadio PC et al (2018) Novel engineered tendon-fibrocartilage-bone composite with cyclic tension for rotator cuff repair. J Tissue Eng Regen Med 12:1690–1701
Nyffeler RW, Schenk N, Bissig P (2021) Can a simple fall cause a rotator cuff tear? Literature review and biomechanical considerations. Int Orthop 45:1573–1582
Plachel F, Jo OI, Ruttershoff K, Andronic O, Ernstbrunner L (2022) A systematic review of long-term clinical and radiological outcomes of arthroscopic and open/mini-open rotator cuff repairs. Am J Sports Med. https://doi.org/10.1177/03635465211073332
Rodeo SA (2007) Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg 16:S191-197
Scanaliato JP, Eckhoff MD, Dunn JC, Czajkowski H, Fink WA, Parnes N (2022) Long-term results of arthroscopic repair of full-thickness traumatic rotator cuff tears in active duty military patients under the age of 40 years. Am J Sports Med 50:2753–2760
Shin MJ, Shim IK, Kim DM, Choi JH, Lee YN, Jeon IH et al (2020) Engineered cell sheets for the effective delivery of adipose-derived stem cells for tendon-to-bone healing. Am J Sports Med 48:3347–3358
Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR (1996) Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 5:383–392
Srinivasan RC, Elhassan BT, Wright TW (2021) Rotator cuff repair and reconstruction. J Hand Surg Am 46:493–500
Tanaka S, Gotoh M, Tanaka K, Mitsui Y, Nakamura H, Ozono H et al (2021) Functional and structural outcomes after retears of arthroscopically repaired large and massive rotator cuff tears. Orthop J Sports Med 9:23259671211035750
Teunis T, Lubberts B, Reilly BT, Ring D (2014) A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elbow Surg 23:1913–1921
Thangarajah T, Henshaw F, Sanghani-Kerai A, Lambert SM, Pendegrass CJ, Blunn GW (2017) Supraspinatus detachment causes musculotendinous degeneration and a reduction in bone mineral density at the enthesis in a rat model of chronic rotator cuff degeneration. Shoulder Elbow 9:178–187
Waldorff EI, Lindner J, Kijek TG, Downie BK, Hughes RE, Carpenter JE et al (2011) Bone density of the greater tuberosity is decreased in rotator cuff disease with and without full-thickness tears. J Shoulder Elbow Surg 20:904–908
Wang Z, Liu X, Davies MR, Horne D, Kim H, Feeley BT (2018) A Mouse model of delayed rotator cuff repair results in persistent muscle atrophy and fatty infiltration. Am J Sports Med 46:2981–2989
Wong MW, Qin L, Lee KM, Leung KS (2009) Articular cartilage increases transition zone regeneration in bone-tendon junction healing. Clin Orthop Relat Res 467:1092–1100
Yakacki CM, Poukalova M, Guldberg RE, Lin A, Saing M, Gillogly S et al (2010) The effect of the trabecular microstructure on the pullout strength of suture anchors. J Biomech 43:1953–1959
Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H et al (2010) Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 19:116–120
Yu Y, Zhou Y, Cheng T, Lu X, Yu K, Zhou Y et al (2016) Hypoxia enhances tenocyte differentiation of adipose-derived mesenchymal stem cells by inducing hypoxia-inducible factor-1alpha in a co-culture system. Cell Prolif 49:173–184
Zhang MT, Liu JX, Yang ZT, Liu T, Zhang BR, An LP et al (2022) Early efficacy analysis on arthroscopic autologous osteochondral grafting in the treatment of recurrent anterior shoulder dislocation. Zhongguo Gu Shang 35:233–237
Zhang W, Wang N, Yang M, Sun T, Zhang J, Zhao Y et al (2022) Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J Orthop Translat 33:41–54
Zhao J, Luo M, Pan J, Liang G, Feng W, Zeng L et al (2021) Risk factors affecting rotator cuff retear after arthroscopic repair: a meta-analysis and systematic review. J Shoulder Elbow Surg 30:2660–2670
Zou J, Bai B, Yao Y (2018) Progress of co-culture systems in cartilage regeneration. Expert Opin Biol Ther 18:1151–1158
Acknowledgements
The authors thank the financial support of the Second Hospital of Lanzhou University, “Cuiying Technology Innovation” program, clinical top-notch technology research project, clinical study of autologous osteochondral transplantation for the treatment of recurrent shoulder dislocation in young adults, and the Gansu Provincial Department of Science and Technology, Natural Science Foundation Project, Study on the mechanism of autologous osteochondral transplantation in the treatment of recurrent shoulder dislocation.
Funding
This work was supported by the The Second Hospital of Lanzhou University, “Cuiying Technology Innovation” program, clinical top-notch technology research project, CY2019-BJ04, clinical study of autologous osteochondral transplantation for the treatment of recurrent shoulder dislocation in young adults, and the Gansu Provincial Department of Science and Technology, Natural Science Foundation Project, 20JR10RA723, Study on the mechanism of autologous osteochondral transplantation in the treatment of recurrent shoulder dislocation.
Author information
Authors and Affiliations
Contributions
MZ and LD collected the data and wrote the article. XY and JJ revised the article. MZ, JL, JZ and YJ designed the study. MZ, TL and ZY performed the rabbits experiment. LD prepared figures and tables. All authors contributed towards data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors, their immediate families and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Ethical approval
This study was approved by the local ethics committee (the Second Hospital of Lanzhou University, Approval No. D2021-016).
Informed consent
No application.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Deng, L., Zhou, J. et al. Combination of autologous osteochondral and periosteum transplantation effectively promotes fibrocartilage regeneration at the tendon–bone junction of the rotator cuff in rabbits. Knee Surg Sports Traumatol Arthrosc 31, 1953–1962 (2023). https://doi.org/10.1007/s00167-022-07250-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-022-07250-6