Abstract
Purpose
Platelet-rich plasma (PRP) represents a highly profitable biological therapy. Platelet-rich plasma is widely used to treat musculoskeletal disorders despite mixed evidence of its efficacy. As evidenced by literature from other domains, industry funding may influence the results of clinical trials. The objective of the current study was to determine the association between industry funding and positive results for randomized controlled trials (RCTs) assessing the efficacy of PRP in musculoskeletal disorders.
Methods
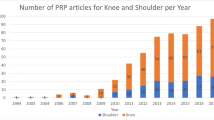
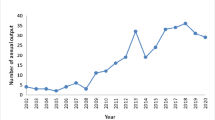
A search of four databases was conducted. Included studies were RCTs comparing PRP to any non-PRP comparator in adults (18 years old or over) with musculoskeletal disorders and had full text available in English. Studies were excluded if they were published before 2016 or were non-human trials. A multivariate binomial logistic regression model was created to explore predictors of statistically significant findings. Covariates included the presence of industry funding, sample size, and length of study follow-up. 1440 records were screened with 87 trials included in the final analysis.
Results
Of the 87 studies, 61 (70%) reported a statistically significant primary outcome. The presence of industry funding was not predictive of a statistically significant primary outcome [OR = 0.36, 95% CI 0.096–1.36, (n.s.)]. Studies that did not state whether industry funding was present had a higher chance of reporting a statistically significant primary outcome (OR = 3.61, 95% CI 1.1–11.9, p = 0.035). Sample size and length of follow-up were not predictive of a statistically significant primary outcome.
Conclusion
The results of the current study conclude that industry funding had no impact on the reporting of positive results for RCTs investigating PRP in musculoskeletal disorders. However, not disclosing sources of funding was associated with a higher likelihood of reporting positive results. The results of trials that fail to disclose funding sources should be interpreted with caution in the PRP literature.
Level of evidence
I.
Similar content being viewed by others
Abbreviations
- RCT:
-
Randomized controlled trial
- PRP:
-
Platelet-rich plasma
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- PROM:
-
Patient reported outcome measure
- CoI:
-
Conflict of interest
References
Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC (2021) Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med 49:249–260
Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A (2021) Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA 326(20):2021–2030
Brophy RH, Fillingham YA (2022) AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty). J Am Acad Orthop Surg 30:e721–e729
Buchkowsky SS, Jewesson PJ (2004) Industry sponsorship and authorship of clinical trials over 20 years. Ann Pharmacother 38:579–585
Buffel du Vaure C, Boutron I, Perrodeau E, Ravaud P (2014) Reporting funding source or conflict of interest in abstracts of randomized controlled trials, no evidence of a large impact on general practitioners’ confidence in conclusions, a three-arm randomized controlled trial. BMC Med 12:1–9
Chaudhry S, Schroter S, Smith R, Morris J (2002) Does declaration of competing interests affect readers’ perceptions? A randomised trial. BMJ 325:1391–1392
Dhillon MS, Behera P, Patel S, Shetty V (2014) Orthobiologics and platelet rich plasma. Indian J Orthop 48:1–9
Ehrenfest DMD, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trends Biotechnol 27:158–167
Ekhtiari S, Gazendam A, Nucci N, Kruse C, Bhandari M (2020) The Fragility of statistically significant findings from randomized controlled trials in hip and knee arthroplasty. J Arthroplasty 36(6):2211–2218
Engebretsen L, Steffen K, Alsousou J, Anitua E, Bachl N, Devilee R, Everts P, Hamilton B, Huard J, Jenoure P (2010) IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br J Sports Med 44:1072–1081
Fundytus A, Wells JC, Sharma S, Hopman WM, Del Paggio JC, Gyawali B, Mukherji D, Hammad N, Pramesh CS, Aggarwal A (2022) Industry funding of oncology randomised controlled trials: implications for design, results and interpretation. Clin Oncol 34:28–35
Gazendam AM, Slawaska-Eng D, Nucci N, Bhatt O, Ghert M (2022) The impact of industry funding on randomized controlled trials of biologic therapies. Medicines (Basel) 9(3):18
Hao Q, Devji T, Zeraatkar D, Wang Y, Qasim A, Siemieniuk RA, Vandvik PO, Lähdeoja T, Carrasco-Labra A, Agoritsas T (2019) Minimal important differences for improvement in shoulder condition patient-reported outcomes: a systematic review to inform a BMJ rapid recommendation. BMJ Open 9:e028777
John LK, Loewenstein G, Marder A, Callaham ML (2019) Effect of revealing authors’ conflicts of interests in peer review: randomized controlled trial. BMJ 367:l5896
Jones IA, Togashi RC, Thomas Vangsness C (2018) The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med 11:558–565
Kaushik A, Kumaran MS (2020) Platelet-rich plasma: the journey so far! Indian Dermatol Online J 11:685
Khan NR, Saad H, Oravec CS, Rossi N, Nguyen V, Venable GT, Lillard JC, Patel P, Taylor DR, Vaughn BN (2018) A review of industry funding in randomized controlled trials published in the neurosurgical literature—the elephant in the room. Neurosurgery 83:890–897
Koucheki R, Gazendam AM, Perera JR, Griffin A, Ferguson P, Wunder J, Tsoi K (2021) Assessment of risk of bias in osteosarcoma and Ewing’s sarcoma randomized controlled trials: a systematic review. Curr Oncol 28:3771–3794
Leopold SS, Warme WJ, Braunlich EF, Shott S (2003) Association between funding source and study outcome in orthopaedic research. Clin Orthop Relat Res 415:293–301
Lexchin J, Bero LA, Djulbegovic B, Clark O (2003) Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 326:1167–1170
Lindsley CW (2017) New 2016 data and statistics for global pharmaceutical products and projections through 2017. ACS Chem Neurosci 8(8):1635–1636
Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L (2017) Industry sponsorship and research outcome. Cochrane Database Syst Rev 2(2):MR000033
Mehrabani D, Seghatchian J, Acker JP (2019) Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus Apher Sci 58:102675
Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC (2014) Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev 4:CD010071
Nesello PFT, Baroni AC, da Silva SL (2020) Level of evidence and industry sponsorship associated with favorable outcomes in publications on platelet-rich-plasma therapy in musculoskeletal disorders. Rev Bras Ortop (Sao Paulo) 55:263–268
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5:210
Patricios J, Harmon KG, Drezner J (2022) PRP use in sport and exercise medicine: be wary of science becoming the sham. Br J Sports Med 56:66–67
Rachul C, Rasko JE, Caulfield T (2017) Implicit hype? Representations of platelet rich plasma in the news media. PLoS One 12:e0182496
Rossi LA, Murray IR, Chu CR, Muschler GF, Rodeo SA, Piuzzi NS (2019) Classification systems for platelet-rich plasma. Bone Joint J 101:891–896
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 11:1–8
Schwarz A (2009, February 16) A Promising Treatment for Athletes, in Blood. The New York Times. https://www.nytimes.com/2009/02/17/sports/17blood.html. Accessed 13 Apr 2022
Google Sheets (2022) Google. https://www.google.com/sheets/about/. Accessed 27 Jan 2022
ClinicalTrials.gov (2022) U.S. National Library of Medicine, Bethesda. https://www.clinicaltrials.gov/. Accessed 25 Mar 2022
Rotator Cuff Injuries - Clinical Practice Guideline (CPG) | American Academy of Orthopaedic Surgeons (2019). https://www.aaos.org/globalassets/quality-and-practice-resources/rotator-cuff/rotator-cuff-cpg-final-12-20-19.pdf. Accessed 16 Apr 2022
Funding
No funding was received in preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to conception and design, acquisition of data, or analysis and interpretation of data for this manuscript. All authors were involved in the drafting or revising of the manuscript and have given it final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
MB receives institutional support from the Canadian Institute of Health Research, National Institute of Health, Michael DeGroote Institute for Pain Research and Care and Physicians’ Services Incorporated Foundation.
Ethical approval
No ethical approval was required.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chou, K., Gazendam, A., Vemulakonda, J. et al. Failure to disclose industry funding impacts outcomes in randomized controlled trials of platelet-rich plasma. Knee Surg Sports Traumatol Arthrosc 31, 626–631 (2023). https://doi.org/10.1007/s00167-022-07118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-022-07118-9