Abstract
Purpose
The aim of this two-centre RCT was to compare pre- and post-operative radiological, clinical and functional outcomes between patient-specific instrumentation (PSI) and conventional instrumented (CI) unicompartmental knee arthroplasty (UKA). It was hypothesised that both alignment methods would have comparable post-operative radiological, clinical and functional outcomes.
Methods
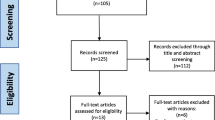
One hundred and twenty patients were included, and randomly allocated to the PSI or the CI group. Outcome measures were peri-operative outcomes (operation time, length of hospital stay and intra-operative changes of implant size) and post-operative radiological outcomes including the alignment of the tibial and femoral component in the sagittal and frontal plane and the hip–knee–ankle-axis (HKA-axis), rate of adverse events (AEs) and patient-reported outcome measures (PROMs) pre-operatively and at 3, 12 and 24 months post-operatively.
Results
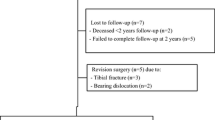
There was a statistically significant difference (p < 0.05) in alignment of the femoral component in the frontal plane in favour of the CI method. No statistically significant differences were found for the peri-operative data or in the functional outcome at 2-year follow-up. In the PSI group, the approved implant size of the femoral component was correct in 98.2% of the cases and the tibial component was correct in 60.7% of the cases. There was a comparable rate of AEs: 5.1% in the CI and 5.4% in the PSI group.
Conclusion
The PSI method did not show an advantage over CI in regard of positioning of the components, nor did it show an improvement in clinical or functional outcome. We conclude that the possible advantages of PSI do not outweigh the costs of the MRI scan and the manufacturing of the PSI.
Level of evidence
Randomised controlled trial, level I.



Similar content being viewed by others
Abbreviations
- ACL:
-
Anterior cruciate ligament
- AE:
-
Adverse event
- AP:
-
Anterior–posterior
- BMI:
-
Body mass index
- CI:
-
Conventional instrumented
- CT:
-
Computed tomography
- EQ-5D-VAS:
-
EuroQuol-5D Visual Analogue Scale
- FJS:
-
Forgotten Joint Score
- HKA-axis:
-
Hip–knee–ankle-axis
- ICD:
-
Implantable cardioverter-defibrillator
- MRI:
-
Magnetic resonance imaging
- NRS-pain:
-
Numeric Rating Scale for pain
- OKS:
-
Oxford Knee Score
- PROMs:
-
Patient-reported outcome measures
- PSI:
-
Patient-specific instrumentation
- RCT:
-
Randomised controlled trials
- SD:
-
Standard deviation
- TKA:
-
Total knee arthroplasty
- UKA:
-
Unicompartmental knee arthroplasty
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index
References
Alvand A, Khan T, Jenkins C, Rees JL, Jackson WF, Dodd CAF, Murray DW, Price AJ (2018) The impact of patient-specific instrumentation on unicompartmental knee arthroplasty: a prospective randomised controlled study. Knee Surg Relat Res 26:1662–1670
Badawy M, Espehaug B, Indrekvam K, Havelin L, Furnes O (2014) Higher revision risk for unicompartmental knee arthroplasty in low-volume hospitals: data from 5791 cases in the Norwegian Arthroplasty Register. Acta Orthop 85(4):342–347
Beard DJ, Davies LJ, Cook JA, MacLennan G, Price A, Kent S, Hudson J, Carr A, Leal J, Campbell H, Fitzpatrick R, Arden N, Murray D, Campbell MK (2019) The clinical and cost-effectiveness of total versus partial knee replacement in patients with medial compartment osteoarthritis (TOPKAT): 5 year outcomes of a rondomised controlled trial. Lancet 394(10200):746–756
Bell SW, Stoddard J, Bennett C, London NJ (2014) Accuracy and early outcomes in medial unicompartmental knee arthroplasty performed using patient specific instrumentation. Knee 21(S1):S33–S36
Bellamy N, Burchanan WW, Goldsmith CH et al (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Biomet. Oxford partial knee—microplasty instrumentation: surgical technique (2013). http://www.zimmerbiomet.com/content/dam/zimmer-biomet/medical-professionals/000-surgical-techniques/knee/oxford-partial-knee-microplasty-instrumentation-surgical-technique.pdf. Accessed 15 Oct 2020
Bolink SAAN, Grimm B, Heyligers IC (2015) Patient-reported outcome measures versus inertial performance-based outcome measures: a prospective study in patients undergoing primary total knee arthroplasty. Knee 22(6):618–623
Boonen B, Schotanus MG, Kort NP (2012) Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop 83:387–393
Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C (2013) A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J 95-B(3):354–359
Chatellard R, Sauleau V, Colmar M, Robert H, Raynaud G, Brilhault J (2013) Medial unicompartmental knee arthroplasty: does tibial component position influence clinical outcomes and arthroplasty survival? Orthop Traumatol Surg Res 99(4 SUPPL):S219–S225
Chotanaphuti T, Wangwittayakul V, Khuangsirikul S, Foojareonyos T (2014) The accuracy of component alignment in custom cutting blocks compared with conventional total knee arthroplasty instrumentation: prospective control trial. Knee 21(1):185–188
Crawford DA, Berend KR, Thienpont E (2020) Unicompartmental knee arthroplasty: US and global perspective. Orthop Clin North Am 51(2):147–159
Dao Trong ML, Diezi C, Goerres G, Helmy N (2014) Improved positioning of the tibial component in unicompartmental knee arthroplasty with patient-specific cutting blocks. Knee Surg Sports Traumatol Arthrosc 23:1993–1998
Eckhard L, Munir S, Wood D, Talbot S, Brighton R, Walter B, Baré J (2020) The ceiling effects of patient reported outcome measures for total knee arthroplasty. Orthop Trauma Surg Res. https://doi.org/10.1016/j.otsr.2020.102758
EuroQuol Group (1990) EuroQuol—a new facility for the measurement of health-related quality of life. Health Policy 16(3):199–208
Flury A, Hasler J, Dimitriou D, Antoniadis A, Finsterwald M, Helmy N (2019) Midterm clinical and radiographic outcomes of 115 consecutive patient-specific unicompartmental knee arthroplasties. Knee 26(4):889–896
Haverkamp D, Breugem SJ, Sierevelt IN et al (2005) Translation and validation of the Dutch version of the Oxford 12-item knee questionnaire for knee arthroplasty. Acta Orthop 76:347–352
Jones GG, Logishetty K, Clarke S, Collins R, Jaere M, Harris S, Cobb JP (2018) Do patient-specific instruments (PSI) for UKA allow non-expert surgeons to achieve the same saw cut accuracy as expert surgeons? Arch OrthopTrauma Surg 138:1601–1608
Kalache H, Müller JH, Saffarini M, Gancel E (2020) Patient-specific instrumentation does not improve tibial component coronal alignment for medial UKA compared to conventional instrumentation. J Exp Orthop 7:42
Kamenaga T, Hiranaka T, Kikuchi K, Hida Y, Fuijshiro T, Okamoto K (2018) Influence of tibial component rotation on short-term clinical outcomes in Oxford mobile-bearing unicompartmental knee arthroplasty. Knee 25(6):1222–1230
Kerens B, Leenders AM, Schotanus MGM, Boonen B, Tuinebreijer WE, Emans PJ, Jong B, Kort NP (2017) Patient-specific instrumentation in Oxford unicompartmental knee arthroplasty is reliable and accurate except for the tibial rotation. Knee Surg Sports Traumatol Arthrosc 26(6):1823–1830
Kohn MD, Sassoon AA, Fernando ND (2016) Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop 474(8):1886–1893
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163
Kort NP, Bemelmans YFL, Schotanus MGM (2017) Outpatient surgery for unicompartmental knee arthroplasty is effective and safe. Knee Surg Sports Traumatol Arthrosc 25(9):2659–2667
McCormack HM, Horne DJ, Sheather S (1988) Clinical applications of visual analogue scales: a critical review. Psychol Med 18:1007–1101
Murray DW, Fitzpatrick RK, Pandit H, Beard DJ, Carr AJ, Dawson J (2007) The use of the Oxford hip and knee scores. J Bone Joint Surg Br 89(8):1010–1014
Leenders AM, Schotanus MGM, Wind RJP, Borghans RAP, Kort NP (2018) A high rate of tibial plateau fractures after early experience with patient-specific instrumentation for unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Artrosc 26(11):3491–3498
National Joint Registry for England, Wales and Northern Ireland (2016) 13th annual report
Pandit H, Jenkins C, Beard DJ, Gallagher J, Price AJ, Dodd CAF, Goodfellow JW, Murray DW (2009) Cementless Oxford unicompartmental knee replacement shows reduced radiolucencz at one year. J Bone Surg Br 91(2):185–189
Sanz-Ruiz P, Matas-Diez JA, Carbo-Laso E, Perez-Mañanes R, Vaquero-Martín J (2019) Patient-specific instrument can improve functional and radiographic results during learning curve for Oxford unicompartmental knee arthroplasty. J Knee Surg 32(2):180–185
Schotanus MGM, Schoenmakers DAL, Sollie R, Kort NP (2017) Patient-specific instruments for total knee arthroplasty can accurately predict the component size as used peroperative. Knee Surg Sports Traumatol Arthrosc 25(12):3844–3848
Schotanus MGM, Sollie R, van Haaren EH, Hendrickx RPM, Jansen EJP, Kort NP (2016) A radiological analysis of the difference between MRI- and CT-based patient-specific matched guides for total knee arthroplasty from the same manufacturer. Bone Joint J 98-B(6):786–792
Seeber GH, Kolbow K, Maus U, Kluge A, Lazovic D (2016) Medial unicompartmental knee arthroplasty using patient-specific instrumentation—accuracy of preoperative planning, time saving and cost efficiency. Z Orthop Unfall 154:287–293
Shadid MB, Vinken NS, Marting LN, Wolterbeek N (2016) The Dutch version of the Forgotten Joint Score: test-retesting reliability and validation. Acta Orthop Belg 82:112–118
Wilson HA, Middleton R, Abram SGF, Smith S, Alvand A, Jackson WF, Bottomley N, Hopewell S, Price AJ (2019) Patient relevant outcomes of unicompartmental versus total knee replacement: systematic review and meta-analysis. BMJ 364:1352
Zhang Q, Zhang Q, Guo W et al (2014) The learning curve for minimally invasive Oxford phase 3 unicompartmental knee arthroplasty: cumulative summation test for learning curve (LCCUSUM). J Orthop Surg Res 9:81
Zimmer-biomet and materialise. SurgiCase knee planner: software user manual. https://www.zimmerbiomet.com/content/dam/zimmer-biomet/drive/User%20Manual%20SurgiCase%20Knee%20Planner.pdf Accessed 15.10.2020
Acknowledgements
The authors would like to thank Ms Margreet Boevé, from the Amphia Hospital for her assistance in the data gathering, Ms Madelon Knijf for her collaboration on the radiologic evaluation and Ms Sharmila Thompson-Venkatesan, MD, and Mr Jan Truijen, MD, who served as external readers, and for their comments that greatly improved the manuscript.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
AL gathered the data, wrote the initial draft of the manuscript and managed the study. MS designed the study and analysed the data, and revised the manuscript. JM analysed the data and revised the manuscript. NK, RG and BK conceived the study and revised the manuscript. KK and BB were involved in the drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
NK is a paid consultant for BodyCad, Bioventus, Stryker, Supportho Medical and Zimmer-Biomet. RG is a paid consultant for Zimmer-Biomet. The research foundation of the Amphia Hospital (RG and KK) receives funding from Zimmer-Biomet, Stryker and Mathys.
Ethical approval
This study was approved by the Independent Review Board (IRB, Zuyd Heerlen, the Netherlands; IRBNr. 12T92) and registered online at the Dutch Trial Register (www.trialregister.nl, Nr. NTR4278).
Informed consent
An informed consent was signed by all patients who were enrolled in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leenders, A.M., Kort, N.P., Koenraadt, K.L.M. et al. Patient-specific instruments do not show advantage over conventional instruments in unicompartmental knee arthroplasty at 2 year follow-up: a prospective, two-centre, randomised, double-blind, controlled trial. Knee Surg Sports Traumatol Arthrosc 30, 918–927 (2022). https://doi.org/10.1007/s00167-021-06471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06471-5