Abstract
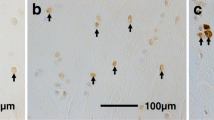
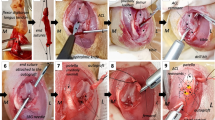
The purpose of this study is to investigate the histological changes and apoptosis of cartilaginous layers in human anterior cruciate ligament (ACL) tibial insertion at different time periods after rupture. By using a core reamer, 35 tibial insertions of ruptured ACLs were obtained during primary ACL reconstructions (number of days after injury: 19–206 days). A histological examination was performed and a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining assay was carried out to detect apoptosis. The average thickness of the cartilage layer, the glycosaminoglycan-stained area and the number of chondrocytes per millimeter decreased with time. The percentage average of TUNEL-positive chondrocytes was 42.0 ± 16.2. The histological degenerative changes of the cartilage layer in the ruptured ACL tibial insertion progressed with time, especially in the first 2 months. Moreover, chondrocyte apoptosis continued from 19 to 206 days after rupture. The results may help elucidate the etiology of the histological changes of the insertion, and may help in devising optimal treatment protocols for ACL injuries if apoptosis is controlled. Moreover, we consider that using a surviving ligament and minimizing a debridement of ACL remnant during ACL reconstruction may be important for ACL reconstruction to maintain cartilage layers in ACL insertions.





Similar content being viewed by others
References
Adams CS, Horton WE Jr (1998) Chondrocyte apoptosis increases with time age in the articular cartilage of adult animals. Anat Rec 250:418–425
Ariga K, Yonenobu K, Nakase T, Hosono N, Okuda S, Meng W, Tamura Y, Yoshikawa H (2003) Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine 28:1528–1533
Arnoczky SP, Tian T, Lavagnino M, Gardner K, Schuler P, Morse P (2002) Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res 20:947–952
Barrack RL, Skinner HB, Buckley SL (1989) Proprioception in the anterior cruciate deficient knee. Am J Sports Med 17:1–6
Benjamin M, Evans EJ, Copp L (1986) The histology of tendon attachments to bone in man. J Anat 149:89–100
Cohen JJ (1993) Apoptosis. Immunol Today 14:126–130
Cooper RR, Misol S (1970) Tendon and ligament insertion: a light and electron microscopic study. J Bone Joint Surg [Am] 52:1–20
Giori NJ, Beaupré GS, Carter DR (1993) Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. J Orthop Res 11:581–591
Golstein P (1997) Controlling cell death. Science 275:1081–1082
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA (1995) Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res 13:907–914
Haapala J, Arokoski JPA, Hyttinen MM, Lammi M, Tammi M, Kovanen V, Helminen HJ, Kiviranta I (1999) Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop 362:218–229
Jurvelin J, Kiviranta I, Tammi M, Helminen JH (1986) Softening of canine articular cartilage after immobilization of the knee joint. Clin Orthop 207:246–252
Kanda T, Ochi M, Ikuta Y (1998) Adverse effects on rabbit anterior cruciate ligament after knee immobilization: changes in permeability of horseradish peroxidase. Arch Orthop Trauma Surg 117:307–311
Keira M, Yasuda K, Kaneda K, Yamamoto N, Hayashi K (1996) Mechanical properties of the anterior cruciate ligament chronically relaxed by elevation of the tibial insertion. J Orthop Res 14:157–166
Klein L, Player JS, Heiple KG, Bahniuk E, Goldberg VM (1982) Isotopic evidence for resorption of soft tissues and bone in immobilized dogs. J Bone Joint Surg [Am] 64:225–230
Murray MM, Martin SD, Martin TL, Spector M (2000) Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg [Am] 82:1387–1397
Murrel GAC, Maddali S, Horovitaz L, Okaley SP, Warren RF (2001) The effects of time course after anterior cruciate ligament injury in correlation with meniscal and cartilage loss. Am J Sports Med 29:9–14
Nabeshima Y, Grood ES, Sakurai A, Herman JH (1996) Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J Orthop Res 14:123–130
Nawata K, Minamizawa T, Yamashita Y, Teshima R (2002) Development of the attachment zones in the rat anterior cruciate ligament: changes in the distribution of proliferating cells and fibrillar collagen during postnatal growth. J Orthop Res 20:1339–1344
Newton PO, Woo SL-Y, MacKenna DA, Akeson WH (1995) Immobilization of the knee joint alters the mechanical and ultrastructural properties of the rabbit anterior cruciate ligament. J Orthop Res13:191–200
Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lauyon LE (2003) Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 284:C934–C943
Noyes FR (1977) Functional properties of knee ligaments and alterations induced by immobilization: a correlative biomechanical and histological study in primates. Clin Orthop 123:210–242
Noyes FR, DeLucas JL, Torvik PJ (1974) Biomechanics of anterior cruciate ligament failure: an analysis of strain-rate sensitivity and mechanisms of failure in primates. J Bone Joint Surg [Am] 56:236–253
Ochi M, Adachi N, Deie M, Kanaya A (2006) Anterior cruciate ligament augmentation procedure with a 1-incision technique: anteromedial bundle or posterolateral bundle reconstruction. Arthroscopy 22:463.e1–465
Sabatini M, Rolland G, Léonce S, Thomas M, Lesur C, Pérez V, de Nanteuil G, Bonnet J (2000) Effects of ceramide on apoptosis, proteoglycan degradation, and matrix metalloproteinase expression in rabbit articular cartilage. Biochem Biophys Res Commun 267:438–444
Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE (2000) Overuse activity injuries the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg 9:79–84
Spindler KP, Clark SW, Nanney LB, Davidson JM (1996) Expression of collagen and matrix metalloproteinases in ruptured human anterior cruciate ligament: an in situ hybridization study. J Orthop Res 14:857–861
Tuoheti Y, Itoi E, Pradhan RL, Wakabayashi I, Takahashi S, Minagawa H, Kobayashi M, Okada K, Shimada Y (2005) Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg 14:535–541
Woo SLY, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH (1987) The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg [Am] 69:1200–1211
Yuan J, Murrell GAC, Wei AQ, Wang MX (2002) Apoptosis in rotator cuff tendonopathy. J Ortop Res 20:1372–1379
Acknowledgements
The authors wish to thank Dr. Akihiro Kanamori and Ms. Jennifer Kanamori for their suggestions. The authors thank the Core Research for Evolutional Science and Technology (CREST) Program, and the Leading Project (LP) of the Ministry of Education, Culture, Sports, Science and Technology of Japan for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mutsuzaki, H., Sakane, M., Ikeda, K. et al. Histological changes and apoptosis of cartilage layer in human anterior cruciate ligament tibial insertion after rupture. Knee Surg Sports Traumatol Arthrosc 15, 602–609 (2007). https://doi.org/10.1007/s00167-006-0264-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-006-0264-x