Abstract
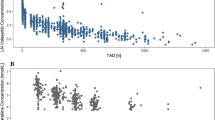
The malononitrilamide FK778 is a leflunomide analogue with a shorter half-life than leflunomide. Groups of cynomolgus monkeys were treated orally with various doses of FK778 once daily for 7 days: group A, 10 mg/kg (n=4); group B, 5 mg/kg (n=3); and group C, one single loading dose of 20 mg/kg followed by 5 mg/kg once daily (n=2). Trough plasma concentration of FK778 was measured by HPLC. Lymphocyte proliferation and expression of T-cell activation surface antigens were assessed by flow cytometry. In group A, trough plasma concentration of FK778 reached steady state at 48 h. After 7 days, lymphocyte proliferation was 23±7.4% (mean ± SEM) and expression of CD71, CD25, CD11a and CD95 on T cells was less than 50% of pre-treatment baseline values. In group B, trough plasma levels of FK778 did not reach steady state, but dropped to near-zero levels after 3 days and on day 7 and lymphocyte proliferation and T-cell surface antigen expression were not different from pre-treatment baseline values. In group C, FK778 trough levels did not reach steady state, but drug exposure was evident over the entire period of treatment, and on day 7, lymphocyte proliferation was 11.4±8.6% of pre-treatment baseline values. We conclude that FK778 inhibits lymphocyte proliferation and expression of T-cell activation antigens in vivo in non-human primates after 1 week of treatment. These effects are related to the total drug exposure over the time of treatment. At doses lower than 10 mg/kg daily, FK778 is cleared from the circulation between the dosing intervals, thus failing to exert its inhibitory effects on immune functions.



Similar content being viewed by others
References
Barten MJ, Gummert JF, van Gelder T, Shorthouse R, Morris RE (2001) Flow cytometric quantitation of calcium-dependent and -independent mitogen-stimulation of T cell functions in whole blood: inhibition by immunosuppressive drugs in vitro. J Immunol Methods 253:95–112
Bîrsan T, Dambrin C, Klupp J, Patz JD, Shorthouse R, Morris RE (2001) Ex vivo evaluation of the immunosuppressive effect of the leflunomide derivative FK778 on whole blood lymphocytes of non-human primates. Am J Transplant 1 [Suppl 1]: 439
Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF (1995) Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373:441–444
Cherwinski HM, Byars N, Ballaron SJ, Nakano GM, Young JM, Ransom JT (1995) Leflunomide interferes with pyrimidine nucleotide biosynthesis. Inflamm Res 44:317–322
Cherwinski HM, Cohn RG, Cheung P, Webster DJ, Xu YZ, Caulfield JP, Young JM, Nakano G, Ransom JT (1995) The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther 275:1043–1049
Chong AS, Finnegan A, Jiang X, Gebel H, Sankary HN, Foster P, Williams JW (1993) Leflunomide, a novel immunosuppressive agent. The mechanism of inhibition of T cell proliferation. Transplantation 55:1361–1366
Chong AS, Rezai K, Gebel HM, Finnegan A, Foster P, Xu X, Williams JW (1996) Effects of leflunomide and other immunosuppressive agents on T cell proliferation in vitro. Transplantation 61:140–145
Chong AS, Huang W, Liu W, Luo J, Shen J, Xu W, Ma L, Blinder L, Xiao F, Xu X, Clardy C, Foster P, Williams JA (1999) In vivo activity of leflunomide: pharmacokinetic analyses and mechanism of immunosuppression. Transplantation 68:100–109
Czech J, Schorlemmer HU, Schwab W (1996) Effect of malononitrilamides on human bone marrow. Transplant Proc 28:3051–3052
Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA (1995) Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem 270:29682–29689
Herrmann ML, Schleyerbach R, Kirschbaum BJ (2000) Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology 47:273–289
Kurrle R, Bartlett R, Ruuth E, Lauffer L, Schorlemmer HU (1996) Malononitrilamides inhibit T- and B-cell responsiveness. Transplant Proc 28:3053–3056
Kyles AE, Gregory CR, Griffey SM, Bernsteen L, Jackson J, Morris RE (2001) Leflunomide analog, MNA-715, plus cyclosporine reduces renal allograft rejection in mismatched dogs. Transplant Proc 33:368–369
Minami Y, Kono T, Miyazaki T, Taniguchi T (1993) The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol 11:245–268
Mladenovic V, Domljan Z, Rozman B, Jajic I, Mihajlovic D, Dordevic J, Popovic M, Dimitrijevic M, Zivkovic M, Campion G (1995) Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum 38:1595–1603
Neckers LM (1991) Regulation of transferrin receptor expression and control of cell growth. Pathobiology 59:11–18
Qi Z, Simanaitis M, Ekberg H (1998) Malononitrilamides 715 and 279 prevent accelerated cardiac allograft rejection synergistically with cyclosporin A in presensitized rats. Transpl Immunol 6:94–100
Qi Z, Simanaitis M, Ekberg H (1999) Malononitrilamides and tacrolimus additively prevent acute rejection in rat cardiac allografts. Transpl Immunol 7:169–175
Ruckemann K, Fairbanks LD, Carrey EA, Hawrylowicz CM, Richards DF, Kirschbaum B, Simmonds HA (1998) Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem 273:21682–21691
Schorlemmer HU, Kurrle R (1998) Hyperacute skin allograft rejection in presensitized rats is abrogated by malononitrilamides. Transplant Proc 30:963–967
Schorlemmer HU, Kurrle R (1998) Malononitrilamides reduce IgM and IgG xenoantibodies and prolong skin xenograft survival in a mouse-to rat model. Transplant Proc 30:976–979
Schorlemmer HU, Schwab W, Ruuth E, Kurrle R (1996) Acute skin graft rejection can be prevented and treated in rat models by malononitrilamides. Transplant Proc 28:3048–3050
Schorlemmer HU, Bartlett RR, Kurrle R (1997) Analogues of leflunomide's primary metabolite, the malononitrilamides, prevent the development of graft-versus-host disease. Transplant Proc 29:1298–1301
Schorlemmer HU, Kurrle R, Bartlett RR (1997) The new immunosuppressants, the malononitrilamides MNA 279 and MNA 715, inhibit various graft-vs.-host diseases (GvHD) in rodents. Drugs Exp Clin Res 23:167–173
Silva HT Jr, Morris RE (1997) Leflunomide and malononitrilamides. Exp Opin Invest Drugs 6:51–64
Silva HT, Cao W, Shorthouse R, Morris RE (1996) Mechanism of action of leflunomide: in vivo uridine administration reverses its inhibition of lymphocyte proliferation. Transplant Proc 28:3082–3084
Slauson SD, Silva HT, Sherwood SW, Morris RE (1999) Flow cytometric analysis of the molecular mechanisms of immunosuppressive action of the active metabolite of leflunomide and its malononitrilamide analogues in a novel whole blood assay. Immunol Lett 67:179–183
Taniguchi T, Minami Y (1993) The IL-2/IL-2 receptor system: a current overview. Cell 73:5–8
Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S (1990) The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol 144:4579–4586
Van Seventer GA, Shimizu Y, Shaw S (1991) Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol 3:294–303
Xu X, Williams JW, Bremer EG, Finnegan A, Chong AS (1995) Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem 270:12398–12403
Acknowledgements
This research was supported by grants from the Max Kade Foundation, the Austrian Science Fund (FWF), the French Society of Transplantation, the Deutsche Forschungsgemeinschaft, the Ralph and Marian Falk Trust, the Hedco Foundation, and Fujisawa Healthcare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bîrsan, T., Dambrin, C., Klupp, J. et al. In vivo pharmacokinetic and pharmacodynamic evaluation of the malononitrilamide FK778 in non-human primates. Transpl Int 16, 354–360 (2003). https://doi.org/10.1007/s00147-003-0591-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00147-003-0591-5