Abstract.
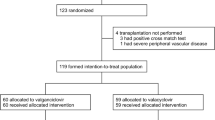
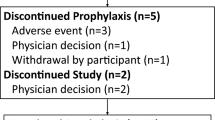
Oral ganciclovir and valacyclovir reduce the incidence of cytomegalovirus (CMV) disease after renal transplantation (RTx). Our study was designed to compare the efficacy, costs, and safety of oral ganciclovir and valacyclovir in the prophylaxis of CMV disease over the first 6 months after RTx. A total of 38 patients was randomized to 3-month treatment with either oral ganciclovir (1 g t.i.d., n=14, GAN group) or oral valacyclovir (2 g q.i.d., n=12, VAL group). A third group (C, n=12) received no prophylaxis. The patients were monitored by CMV-nested PCR in whole blood. No differences were found between the groups in their demographic characteristics, immunosuppressive protocols, or donor and recipient CMV serology. Thirty-six out of 38 (94.7%) recipients were CMV-seropositive. Over the 6-month post-RTx period, there were 13 episodes of CMV disease in eight (66.7%) patients of the C group compared with none in the GAN and VAL groups (P=0.0005, GAN vs C; P=0.001, VAL vs C). The incidence of CMV viremia was 30.8%, 50.0%, and 91.7% in the GAN, VAL, and C groups, respectively (P=0.004, GAN vs C; P=0.07, VAL vs C; P=NS, GAN vs VAL). Treatment failure (death, graft loss, CMV disease, or withdrawal from study) occurred in 14.3%, 0% and 66.7% in the GAN, VAL, and C groups, respectively (P=0.014, GAN vs C; P=0.001, VAL vs C; P=NS, GAN vs VAL). The average CMV-associated costs per patient (in 2001 euros) were 2,449±1,178, 2,485±581, and 4,259±4,616 in the GAN, VAL, and C groups, respectively. Ganciclovir and valacyclovir were well tolerated, with ganciclovir having had to be withdrawn shortly in one patient only because of thrombocytopenia. In conclusion, oral ganciclovir and valacyclovir are equally safe and effective in the prophylaxis of CMV disease after RTx. Both are cost-effective and help reduce CMV-associated costs by some 40% compared with patients without prophylaxis.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
About this article
Cite this article
Reischig, T., Opatrny, K., Bouda, M. et al. A randomized prospective controlled trial of oral ganciclovir versus oral valacyclovir for prophylaxis of cytomegalovirus disease after renal transplantation. Transpl Int 15, 615–622 (2002). https://doi.org/10.1007/s00147-002-0475-0
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00147-002-0475-0