Abstract
Purpose
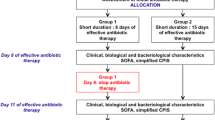
Duration of antibiotic therapy for ventilator-associated pneumonia (VAP) due to non-fermenting Gram-negative bacilli (NF-GNB), including Pseudomonas aeruginosa (PA) remains uncertain. We aimed to assess the non-inferiority of a short duration of antibiotics (8 days) vs. prolonged antibiotic therapy (15 days) in VAP due to PA (PA-VAP).
Methods
We conducted a nationwide, randomized, open-labeled, multicenter, non-inferiority trial to evaluate optimal duration of antibiotic treatment in PA-VAP. Eligible patients were adults with diagnosis of PA-VAP and randomly assigned in 1:1 ratio to receive a short-duration treatment (8 days) or a long-duration treatment (15 days). A pre-specified analysis was used to assess a composite endpoint combining mortality and PA-VAP recurrence rate during hospitalization in the intensive care unit (ICU) within 90 days.
Results
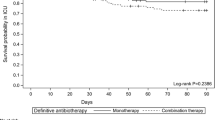
The study was stopped after 24 months due to slow inclusion rate. In intention-to-treat population (n = 186), the percentage of patients who reached the composite endpoint was 25.5% (N = 25/98) in the 15-day group versus 35.2% (N = 31/88) in the 8-day group (difference 9.7%, 90% confidence interval (CI) −1.9%–21.2%). The percentage of recurrence of PA-VAP during the ICU stay was 9.2% in the 15-day group versus 17% in the 8-day group. The two groups had similar median days of mechanical ventilation, of ICU stay, number of extra pulmonary infections and acquisition of multidrug-resistant (MDR) pathogens during ICU stay.
Conclusions
Our study failed to show the non-inferiority of a short duration of antibiotics in the treatment of PA-VAP, compared to a long duration. The short duration strategy may be associated to an increase of PA-VAP recurrence. However, the lack of power limits the interpretation of this study.


Similar content being viewed by others
Change history
21 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00134-022-06776-0
References
Kalil AC, Metersky ML, Klompas M et al (2016) Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63(5):e61–e111. https://doi.org/10.1093/cid/ciw353
Torres A, Niederman MS, Chastre J et al (2017) International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. https://doi.org/10.1183/13993003.00582-2017
Leone M, Bouadma L, Bouhemad B et al (2018) Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med 37(1):83–98. https://doi.org/10.1016/j.accpm.2017.11.006
Hurley JC (2019) Worldwide variation in Pseudomonas associated ventilator associated pneumonia. A meta-regression. J Crit Care 51:88–93. https://doi.org/10.1016/j.jcrc.2019.02.001
Chastre J, Wolff M, Fagon JY et al (2003) Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290(19):2588. https://doi.org/10.1001/jama.290.19.2588
Pugh R, Grant C, Cooke RPD, Dempsey G (2015) Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 8:CD007577. https://doi.org/10.1002/14651858.CD007577.pub3
Combes A, Luyt CE, Fagon JY, Wolff M, Trouillet JL, Chastre J (2007) Early predictors for infection recurrence and death in patients with ventilator-associated pneumonia. Crit Care Med 35(1):146–154. https://doi.org/10.1097/01.CCM.0000249826.81273.E4
Bouglé A, Foucrier A, Dupont H et al (2017) Impact of the duration of antibiotics on clinical events in patients with Pseudomonas aeruginosa ventilator-associated pneumonia: study protocol for a randomized controlled study. Trials 18(1):37. https://doi.org/10.1186/s13063-017-1780-3
Toulouse E, Lafont B, Granier S, Mcgurk G, Bazin JE (2020) French legal approach to patient consent in clinical research. Anaesth Crit Care Pain Med 39(6):883–885. https://doi.org/10.1016/j.accpm.2020.10.012
European Medicines Agency (2013) Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. Committee for Human Medicinal Products
Planquette B, Timsit JF, Misset BY et al (2013) Pseudomonas aeruginosa ventilator-associated pneumonia. Predictive factors of treatment failure. Am J Respir Crit Care Med 188(1):69–76. https://doi.org/10.1164/rccm.201210-1897OC
Berry DA (1996) Statistics: a Bayesian perspective
Capellier G, Mockly H, Charpentier C et al (2012) Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS ONE 7(8):e41290. https://doi.org/10.1371/journal.pone.0041290
Fekih Hassen M, Ayed S, Ben Sik Ali H, Gharbi R, Marghli S, Elatrous S (2009) Duration of antibiotic therapy for ventilator-associated pneumonia: comparison of 7 and 10 days. A pilot study. Ann Fr Anesth Reanim 28(1):16–23. https://doi.org/10.1016/j.annfar.2008.10.021
Kollef MH, Chastre J, Clavel M et al (2012) A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care 16(6):R218. https://doi.org/10.1186/cc11862
Medina J, Perez Protto S, Paciel D, Pontet J, Saldun P, Berro M (2007) Antibiotic treatment for the ventilator-associated pneumonia: 8 vs 12 days randomized trial preliminary data. In: Proceedings of the 47th interscience conference on antimicrobial agents and chemotherapy, Chicago, IL, 17–20 Sept 2007, p 361
Dimopoulos G, Poulakou G, Pneumatikos IA, Armaganidis A, Kollef MH, Matthaiou DK (2013) Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia: a systematic review and meta-analysis. Chest 144(6):1759–1767. https://doi.org/10.1378/chest.13-0076
Acknowledgements
We wish to thank Moreno Ursino, Ph.D., from the Clinical Research Unit of Robert-Debré hospital (AP-HP), Inserm CIC-EC 1426, for the post hoc Bayesian analysis. The iDIAPASON Trial Investigators: Adrien Bouglé: Hôpitaux Universitaires Pitié-Salpêtrière, APHP, Paris; Julien Amour: Hôpitaux Universitaires Pitié-Salpêtrière, APHP, Paris; Thomas Dessalle: Hôpitaux Universitaires Pitié-Salpêtrière, APHP, Paris; Florence Bellenfant Zegdi : Hôpital Européen Georges Pompidou, APHP, Paris; Bernard Cholley ; Julien Massot: Hôpital Européen Georges Pompidou, APHP, Paris; Jean-Michel Constantin: CHU Clermont-Ferrand, Clermont-Ferrand; Alexandre Demoule; Julien Mayaux : Hôpitaux Universitaires Pitié-Salpêtrière, APHP, Paris; Vincent Dubée: Hôpital Saint-Antoine, APHP, Paris; Hervé Dupont : CHU Amiens, Amiens; Jacques Duranteau : Hôpital Bicêtre, APHP, Paris; Laura Federici : Centre Hospitalier Sud Francilien, Corbeil; Arnaud Foucrier: Hôpital Beaujon, APHP, Paris; Thomas Geeraerts : CHU Toulouse, Toulouse; Céline Guichon : Hôpital Croix Rousse, CHU Lyon, Lyon; Pierre Kalfon : Hôpital Louis Pasteur, CH de Chartres, Chartres; Éric Kipnis: CHRU Lille, Lille; Sigismond Lasocki : CHU Angers, Angers; Jean-Yves Lefrant: CHU Nîmes, Nîmes; Matthieu Legrand : Groupe Hospitalier Lariboisière – Saint Louis, APHP, Paris; Marc Leone: CHU Hôpital Nord, APHM, Marseille; Thomas Lescot : Hôpital Saint-Antoine, APHP, Paris; Bruno Lévy : CHU Nancy Brabois, Nancy; Joël Cousson: CHU Reims, Reims; Philippe Montravers, Sébastien Tanaka: Hôpital Bichat, APHP, Paris; Emmanuel Novy : CHU Nancy Brabois, Nancy; Alexandre Ouattara : CHU Bordeaux, Bordeaux; Jean-François Payen: CHU Grenoble, Grenoble; Walter Picard: Centre Hospitalier de Pau, Pau; Pascale Poète: Hôpitaux Universitaires Pitié-Salpêtrière, APHP, Paris; Julien Pottecher : Nouvel Hôpital Civil, CHRU Strasbourg, Strasbourg; Christophe Quesnel, Muriel Fartoukh: Hôpital Tenon, APHP, Paris; Anoine Tesniere, Mélanie Fromentin: Hôpital Cochin, APHP, Paris; Jean-Jacques Rouby, Qin Lu, Olivier Langeron: Hôpitaux Universitaires Pitié-Salpêtrière, APHP, Paris; Pierre Squara: Clinique Ambroise Paré, Neuilly-sur-Seine; Eric Levesque: Hôpital Henri Mondor, APHP, Créteil; Nicolas Mongardon: Hôpital Henri Mondor, APHP, Créteil. Methodology and biostatistics team: Tabassome Simon, Laurence Berard, Marine Cachanado, Nora Soussi: Unité de Recherche Clinique du GH HUEP (URC-Est), Hôpital Saint-Antoine, APHP, Paris
Funding
The sponsor was Assistance Publique – Hôpitaux de Paris (Département de la Recherche Clinique et du Développement, Clinical Research and Development Department). The research was funded by a grant from Programme Hospitalier de Recherche Clinique—PHRC 2014 (Ministère de la Santé).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A complete list of iDIAPASON Trial Investigators is provided in the Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bouglé, A., Tuffet, S., Federici, L. et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia in adults: a randomized, controlled, open-label trial. Intensive Care Med 48, 841–849 (2022). https://doi.org/10.1007/s00134-022-06690-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-022-06690-5