Abstract
Purpose
A systematic review and meta-analysis was conducted to answer the question ‘In adults with respiratory failure requiring invasive ventilation for more than 24 h, does a weaning strategy with early extubation to non-invasive ventilation (NIV) compared to invasive ventilation weaning reduce all-cause hospital mortality?’
Methods
We included randomised and quasi-randomised controlled trials that evaluated the use of non-invasive ventilation, compared to invasive ventilation, as a weaning strategy in adults mechanically ventilated for at least 24 h. The EMBASE, MEDLINE and Cochrane Central Register of Controlled Trials (CENTRAL) bibliographic databases were searched from inception to February 2018. Bayesian hierarchical models were used to perform the meta-analysis. The primary outcome was mortality at hospital discharge. Secondary outcomes included mortality (30, 60, 90 and 180 days), quality of life, duration of invasive ventilation, weaning failure, length of stay [intensive care unit (ICU) and hospital] and adverse events.
Results
Twenty-five relevant studies involving 1609 patients were included in the quantitative analysis. Studies had moderate to high risk of bias due to risk of performance and detection bias. Mortality at hospital discharge was lower in the NIV weaning group compared to the invasive weaning group [pooled odds ratio (OR) 0.58, 95% highest density interval (HDI) 0.29–0.89]. Subgroup analyses showed lower pooled mortality at hospital discharge rates in NIV weaning than those in the control group in chronic obstructive pulmonary disease (COPD) patients (pooled OR 0.43, 95% HDI 0.13–0.81) and the effect is less certain in the mixed ICU population (pooled OR 0.88, 95% HDI 0.25–1.48). NIV weaning reduced the duration of invasive ventilation in patients [standardised mean difference (SMD) − 1.34, 95% HDI − 1.92 to − 0.77] and ICU length of stay (SMD − 0.70, 95% HDI − 0.94 to − 0.46). Reported rates of ventilator associated pneumonia (VAP) were lower in the NIV group. NIV weaning did not reduce overall hospital length of stay or long-term mortality. There were insufficient data to compare other adverse events and health-related quality of life.
Conclusions
The use of NIV in weaning from mechanical ventilation decreases hospital mortality, the incidence of VAP and ICU length of stay. NIV as a weaning strategy appears to be most beneficial in patients with COPD.
Similar content being viewed by others
Introduction
Mechanical ventilation is used to treat 30–40% of patients admitted to critical care [1, 2]. Duration of invasive ventilation is associated with increased mortality [2]. Successful weaning and liberation from invasive mechanical ventilation is important to improve outcomes in critically ill patients [3]. Current international guidelines recommend daily assessment of readiness for extubation with a spontaneous breathing trial, regular breaks in sedation, early mobilisation and protocolised rehabilitation to help with weaning [4]. However, in common with many critical care interventions, successful liberation from ventilation is a complex process that requires patients to co-operate, breathe without mechanical aid, maintain their airway, expectorate secretions and tolerate ensuing physiological stress. The process of reliably identifying when a patient is ready to be extubated following invasive mechanical ventilation is clinically challenging [5]. Spontaneous breathing trials (SBT) are a commonly used test to assess patients’ readiness to wean [6, 7]. They assess the patients ability to breathe without positive airway pressure support. Most patients are successfully weaned off ventilation following the first SBT, but up to a third of patients fail one or more SBTs, requiring prolonged ventilation and are deemed ‘difficult to wean’ [3, 8]. Whilst modern ventilators have improved with advancing technology, there is a lack of consensus of how best to conduct weaning in this population [5].
Non-invasive ventilation has become a commonly used alternative to invasive ventilation [9]. Extubation to non-invasive ventilation (NIV) following a failed SBT may be an attractive weaning strategy. The benefits of this approach include avoidance of the injurious effects of invasive mechanical ventilation and reduction in sedation requirements and a lower risk of nosocomial pneumonia [3, 8]. The key risk is the potential need for re-intubation, which is associated with an increased risk of mortality [10]. Non-invasive ventilation could, however, prolong the period of weaning if it led to continuation of non-invasive mechanical ventilation longer than would have occurred with an invasive ventilation weaning strategy. Thus uncertainty exists about the most effective strategy. Current European Respiratory Society/American Thoracic Society guidelines recommend that NIV be used as a weaning strategy from invasive ventilation, but this recommendation is limited to hypercapnic respiratory failure patients [11]. These guidelines are based on the 2014 Cochrane review on the use of non-invasive weaning, which included 16 randomised studies and concluded that NIV weaning was superior to invasive weaning with significantly reduced mortality, weaning failures, ventilator associated pneumonia (VAP), intensive care and hospital length of stay and total duration of mechanical ventilation [12]. However, these studies were often conducted in single centres involving small number of patients, limiting the generalisability of their review findings.
On the basis of ongoing clinical uncertainty as to optimum weaning strategy, the UK National Institute of Health Research commissioned the Breathe study in 2013. As the largest randomised controlled trial published to date which addresses this clinical question, the recent publication of the Breathe study provides a timely opportunity to review clinical evidence in this area [13]. The aim of this systematic review and meta-analysis is to evaluate the effect of a weaning strategy of using non-invasive ventilation, compared with ongoing invasive mechanical ventilation, in adult patients that are considered clinically ready for weaning on mortality at hospital discharge and other clinically important outcomes.
Methods
We conducted this systematic review and meta-analysis in accordance with a protocol, registered with PROSPERO (CRD42017076522).
Study eligibility criteria
We included all randomised and quasi-randomised controlled trials that evaluated the use of non-invasive ventilation, compared to invasive ventilation, as a weaning strategy in adults with respiratory failure intubated for at least 24 h. We excluded studies of weaning in the immediate (up to 24 h) postoperative period, or where a comparator group was either standard oxygen therapy or continuous positive airway pressure (CPAP). Quasi-randomised controlled trials were defined as interventional trials where the group allocation was not truly random (e.g. group allocation by day of week). Studies of NIV use after unplanned extubation, as a rescue therapy after failed extubation, and to facilitate tracheostomy weaning were also excluded.
We included studies reported only as abstracts. No date or language restrictions were applied.
Information sources and search strategy
We searched EMBASE, MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL) bibliographic databases (from inception to 2018) using a combination of keywords and MeSH terms in February 2018. An example search strategy is included in the electronic supplementary material (ESM). Additional citations were identified through citation tracking (forward and backward) of eligible studies and relevant systematic review papers (see Supplementary Appendix A in ESM for search strategy).
On search completion and following duplicate removal, two authors (JY/KC) independently assessed the title of each citation and excluded obviously irrelevant titles. A third author (SG) acted as adjudicator where agreement could not be reached. Following title screening, the same independent review process was adopted for the screening of abstracts and full texts.
Data extraction
Data were extracted onto a piloted proforma by one author and checked for accuracy by a second author (JY/KC). Extracted data included study characteristics (e.g. setting, publication year), population characteristics (e.g. proportion of patients with chronic pulmonary disease), characteristics of the intervention and comparator, and outcomes.
Outcomes
The primary review outcome was mortality at hospital discharge. Secondary outcomes were mortality (measured at 30, 60, 90 and 180 days), health-related quality of life, duration of invasive ventilation, weaning failure, intensive care unit (ICU) length of stay, hospital length of stay and adverse events.
We acknowledged that there would likely be variation in the definition of outcomes such as weaning failure, so elected to accept the outcome as defined in each study. For the adverse event of VAP, we excluded studies that reported non-specific pneumonia outcomes, such as nosocomial pneumonia or antibiotic use.
Risk of bias
We assessed risk of bias in individual studies using the Cochrane tool for bias assessment in randomised controlled studies. The assessment was undertaken by two authors (JY/KC). We assessed effects of publication bias on primary outcome of mortality by constructing and visually inspecting a funnel plot of study effect estimates and standard error of log RR.
Synthesis of results
Bayesian hierarchical models were used to perform the random effects meta-analysis to account for between-trial variations in treatment effects, as well as variability within a trial. Bayesian hierarchical models allow for partial pooling of information across trials, and so the Bayesian estimate of the treatment effect for each trial is informed by the results of other trials. This improves estimates of treatment effects where there is little information, and reduces the sensitivity of the estimates to studies with extreme results. We employed the Bayesian approach for its flexibility and ability to model a small number of studies and account for uncertainty in the treatment effect. Bayesian analyses allow previous information about the treatment effect to be incorporated via the prior distribution. This is then updated using the current data to become the posterior distribution, which provides the probability of various estimates of the treatment effect.
Posterior distributions were obtained for the treatment effect for each study and the posterior mean estimate and 95% highest posterior density interval (95% HDI) were used to summarise the Bayesian estimates. HDIs are Bayesian alternatives to confidence intervals and for a 95% HDI there is a 95% probability that the treatment effect falls in this interval. A posterior distribution for the treatment effect across the studies was also obtained from the Bayesian hierarchical model, where the mean represented the pooled effect and the variance of this distribution describes the between-study heterogeneity.
The Bayesian model requires specification of prior distributions for the pooled effect and between-study heterogeneity. We used minimally informative prior distributions for the meta-analysis since we incorporated all relevant previous studies into the meta-analysis and wanted the data from these trials to drive the final inferences. A normal distribution with a large variance, N(0, 106), was used for the pooled treatment effect. A uniform distribution with a lower bound of 1/1000 was used for the between-study standard deviation to ensure positive values; an upper bound of 10 was generally used, apart from the 30-, 60- and 90-day mortality which used an upper bound of 2 due to convergence issues and the small number of studies.
For each outcome, we assumed that the treatment effect for each study was normally distributed and had its own mean and variance (assumed to be equal to the study’s observed variance). For binary outcomes (i.e. hospital mortality, 30-, 60- and 90-day mortality, VAP), we assumed that the logarithms of the odds ratios (OR) were normally distributed. Since most of the studies have a small sample size, and there are zero counts for some studies, a value of 0.5 was added to each of the cells (in the 2 × 2 table that the data formed for each study) so that the log OR could be calculated [14]. For the duration of invasive ventilation and length of stay (LOS; hospital and ICU LOS) outcomes, means and standard deviations from each of the studies were used in the meta-analysis. Studies that report medians and interquartile ranges were not included in the meta-analyses. Effect sizes for the duration/LOS outcomes were calculated as the standardised difference in the means (SMD), i.e. Hedges’ g [15], and were assumed to be normally distributed. The Bayesian hierarchical models did not include covariate adjustment.
The Bayesian meta-analyses were performed using the Markov chain Monte Carlo (MCMC) algorithm and were conducted in R (version 3.4.1) using the rjags package (JAGS version 4.3.0). Three chains were run using the MCMC algorithm, each with 50,000 iterations and a burn-in of 1000 iterations (and a thinning of 10). Convergence of the chains was determined by examining the trace and density plots of the parameters, and the Gelman–Rubin diagnostic [16] (see Supplementary Appendix B in in ESM for code and Appendix C for datasets).
Analysis of subgroups or subsets
We performed subgroup analysis for the majority of outcomes to examine studies by patient population: chronic pulmonary disease (COPD) patients compared with the mixed ICU population. On the basis of a previous systematic review by Burns et al., there might be significantly higher mortality benefit for COPD patients in NIV weaning [17]. Clinical guidelines from Canadian Critical Care Trials Group/Canadian Critical Care Society Non-invasive Ventilation Guidelines Group also suggested that NIV should be used to facilitate early liberation from mechanical ventilation in COPD patients but no recommendation was made for the mixed ICU population due to lack of benefit [18]. We were unable to perform this subgroup analysis for 30-, 60- and 90-day mortality due to limited number of studies.
For the primary outcome, we also performed an additional subgroup analysis to examine studies by intervention type: studies that used protocolised weaning compared with studies where a weaning protocol was not used (or not stated). We defined protocolised weaning as a strategy where, provided there was no contraindication, ventilatory support was reduced by a set amount (e.g. 2 cmH2O) over a set time period (e.g. every 2 h).
We conducted two sensitivity analyses for the primary outcome, which were not defined a priori. Firstly, we repeated our meta-analysis with different priors to examine the impact of our choice of prior on the model and findings of our meta-analysis. We also examined the group of studies that used a failed SBT as a requirement for study entry as this reflects current standard practice.
Results
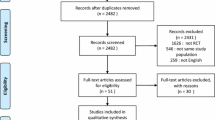
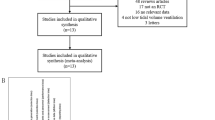
Database searches identified 1508 citations with a further nine citations identified through citation tracking (Fig. 1). Following removal of duplicates and ineligible citations, we included 25 relevant studies involving 1609 patients in the quantitative analysis. Characteristics of included studies are shown in Table 1. The majority of included studies were conducted in Asia (n = 11, 46%) and Europe (n = 8, 32%). Eighteen studies (72%) were reported as single-centre, with only three studies (12%) having more than five centres. Across all studies, the total sample size was 1609 with a median sample size per study of 50 (IQR 30–69). Most studies recruited only patients with chronic respiratory disease (n = 15, 60%), although these studies accounted for only 49% (n = 783) of the total sample. The remaining ten studies (40%) recruited a mixed ICU population. None of the included studies reported outcome data adjusted for baseline patient characteristics.
Studies included patients that were considered potentially suitable for extubation (e.g. acceptable level of consciousness, oxygen requirement and haemodynamic status), although precise inclusion criteria varied between studies. Fourteen studies (56%) used a failed SBT as a trigger for study entry, which excluded patients who would be suitable for immediate extubation. Other studies used completion of the pulmonary infection control (PIC) window (20%) or a specified period of invasive mechanical ventilation. Only six studies described the use of a standardised weaning protocol whilst no details were provided in the other studies.
Risk of bias
The risk of bias across studies varied markedly (Fig. 2). There was a lack of description of randomisation in the majority of studies. The inability to blind caregivers to treatment allocation meant that all studies were considered to be at high risk of performance bias. The majority of the included studies did not describe any strategies to blind outcome assessors from group allocation. Studies were rarely registered, creating a risk of selective reporting. Visual inspection of funnel plot did not reveal important asymmetry or publication bias for mortality outcome.
Outcomes
The primary outcome of hospital mortality was reported in 16 studies [13, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], which included 1156 patients. Overall, hospital mortality was lower in the NIV weaning group compared to the invasive weaning group (pooled OR 0.58, 95% HDI 0.29–0.89) [27]. Subgroup analyses of studies by patient population showed that pooled hospital mortality rates were lower in the NIV arm than those in the control group in patients with COPD (pooled OR 0.43, 95% HDI 0.13–0.81). In the mixed ICU population, pooled hospital mortality rates were similar and there was no evidence of difference in survival observed between the two arms (pooled OR 0.88, 95% HDI 0.25–1.48) but a wide HDI for this outcome made the results less clear-cut for this population (Fig. 3).
Forest plot comparing hospital mortality rates for NIV and invasive weaning, by patient population (COPD vs. mixed ICU population). The estimated odds ratio (OR) from the posterior distribution for each study is shown as a circle, with 95% HDI represented by horizontal lines. The observed OR are given by crosses. The pooled OR estimates (and 95% HDI) are also displayed as the last row for each patient population, and the overall pooled estimate for all studies is displayed as the last row. An OR < 1 means that the intervention is superior
In a subgroup analysis on the primary outcome, we compared the results of studies which had a specified protocol for weaning and studies that did not specify a protocol for weaning. For the protocolised weaning studies, the pooled OR for hospital mortality was 0.59 (95% HDI 0.06, 1.25). For the studies that had no protocol for the weaning, the pooled OR for hospital mortality was 0.60 (95% HDI 0.23, 1.01) (Fig. 4).
Forest plot comparing hospital mortality rates for NIV and invasive weaning, by studies that used protocolised weaning and those that did not. The estimated odds ratio (OR) from the posterior distribution for each study is shown as a circle, with 95% HDI represented by horizontal lines. The observed OR are given by crosses. The pooled OR estimates (and 95% HDI) are also displayed as the last row for each patient population. An OR < 1 means that the intervention is superior
Other mortality outcomes were infrequently reported, such that meta-analyses included only three 30-day studies [13, 36, 37], three 60-day studies [34, 35, 39] and three 90-day studies [13, 19, 38]. In the meta-analyses, the pooled OR for 30-day, 60-day and 90-day mortality was lower in NIV group, but the 95% HDI crossed one for each of these analyses (Supplementary Fig. 5 in ESM). Only one study reported on survival at 180 days and did not find a survival benefit with NIV weaning [13].
Health-related quality of life was reported in only one study and was reported to be similar between NIV weaning and invasive weaning patients [11].
Duration of invasive ventilation was reported to be reduced by NIV weaning in all 19 studies (1171 patients) [13, 19,20,21,22,23,24, 28,29,30,31,32, 34, 36,37,38,39,40,41]. Overall NIV weaning reduced the duration of invasive ventilation duration in patients (SMD − 1.34, 95% HDI − 1.92 to − 0.77). In the subgroup analysis, the duration of invasive ventilation was reduced in both COPD patients (SMD − 1.63, 95% HDI − 2.52 to − 0.80) and the mixed ICU population (SMD − 0.78, 95% HDI − 1.44 to − 0.08) (Supplementary Fig. 6 in ESM).
ICU and hospital LOS were reported in 17 (1094 patients) [13, 19, 21,22,23,24,25, 28, 29, 31,32,33, 36,37,38,39,40,41] and 9 (780 patients) [13, 19, 20, 22, 23, 30, 31, 38, 40] studies, respectively. One study could not be included in the meta-analysis for ICU LOS due to data discrepancies within the paper [42]. Overall NIV weaning reduced LOS on ICU compared to invasive weaning (SMD − 0.70, 95% HDI − 0.94 to − 0.46). In the subgroup analysis, all 11 studies in COPD patients reported reduction in ICU LOS with the use of NIV weaning (SMD − 0.84 days, 95% HDI − 1.10 to − 0.56) (Supplementary Fig. 7 in ESM). Six studies reported reduced ICU LOS but the reduction was smaller (SMD − 0.47, 95% HDI − 0.98 to 0.03) and HDIs were wide in the mixed ICU population. There was also a reduction in hospital LOS overall (SMD − 0.76, 95% HDI − 1.60 to 0.03) which was less striking and the overall pooled estimates did not suggest a reduction in LOS in COPD or the mixed ICU population (Supplementary Fig. 8 in ESM).
Weaning failure was reported in 13 studies [13, 19, 24, 27,28,29, 31, 33, 34, 37, 38, 41, 43]. Marked heterogeneity across studies in the way that weaning failure was defined precluded meta-analysis. The reported OR in 11 studies favoured non-invasive ventilation, although the 95% confidence interval in nine of these studies transected one (Supplementary Fig. 9 in ESM).
For adverse events, we were able to compare VAP which was clearly defined and reported by 14 studies (722 patients) [20,21,22,23,24, 28, 30, 31, 33,34,35,36, 41, 43]. Reported rates of VAP were lower in patients that received NIV weaning across all studies and pooled estimates suggested that NIV lowered VAP rates in COPD and mixed ICU patients (Supplementary Fig. 10 in ESM). There were insufficient data to compare other reported adverse events.
Sensitivity analyses
For the majority of outcomes, there were little differences observed in the results of the meta-analyses from using different priors for the population standard deviation or precision (Supplementary Table 2 in ESM). There were convergence issues in the MCMC algorithms when gamma priors were used for the population precision for the 30-, 60- and 90-day mortality, most likely due to the small number of studies for these measures. More precise priors were also explored for the population mean, but these had little impact on the results (results not shown).
A sensitivity analysis of hospital mortality using results from the nine studies (788 patients) that used a failed SBT as a requirement for study entry found there was no evidence that use of NIV weaning led to a difference in hospital mortality but the wide HDI precluded a definitive statement of the effect on this outcome (pooled OR 0.84, 95% HDI 0.31–1.37) (Supplementary Fig. 11 in ESM).
Discussion
This systematic review included 25 studies with 1609 patients that compared NIV weaning to invasive weaning in a randomised controlled trial. Our systematic review included the largest randomised controlled study conducted to date that addressed this research question. We were able to confirm that mortality at hospital discharge was lower in the NIV weaning group compared to the invasive weaning group. In addition, NIV weaning reduced both the duration of invasive ventilation and ICU LOS. To add to evidence in this area, our results also demonstrated that NIV led to substantially lower mortality at hospital discharge in patients with COPD but there was considerable uncertainty about the effects in the mixed ICU population. In addition, NIV weaning reduced both the duration of invasive ventilation and ICU LOS. In contrast to a previous systematic review, we did not find any difference in survival between patients that received protocolised versus non-protocolised weaning [44].
Our results showed that NIV led to substantially lower hospital mortality in patients with COPD but there was considerable uncertainty about the effects in the mixed ICU population. Compared to patients without COPD, COPD patients are more prone to acute exacerbations and respiratory infections [45, 46]. Prolonged weaning, prolonged mechanical ventilation and tracheotomy use are more common in patients with COPD and even more common in COPD patients with respiratory failure [47]. Since the most common reported causes of death in critically ill patients were refractory multi-organ failure and non-respiratory organ dysfunction, we suggest that the large survival effect seen in COPD patients may reflect the importance of reducing the duration of ventilation and lowering the risk of secondary complications in those with COPD [46, 48, 49]. It is also possible that the magnitude of the effect was magnified by the fact that studies that exclusively recruited COPD patients were mostly single-centred, involved fewer patients and were at increased risk of bias.
A potential limitation of previous reviews was the pooling of studies that used and did not use (or did not report the use of) protocolised weaning strategies. This is important as the use of weaning protocols per se is associated with reduced duration of mechanical ventilation and ICU LOS. In contrast to a previous systematic review, separate subgroup analysis examining protocolised versus non-protocolised weaning (or not stated) did not find additional survival benefit in patients who were weaning using a protocol [50]. However, description of protocol and their adherence were not always well described (Table 1). The point estimates for the odds ratio are similar for studies which used protocolised weaning and those that did not. This finding suggests that the effects of non-invasive weaning are not limited to whether weaning protocols are used.
No long-term survival benefit of NIV was demonstrated at 30, 60 or 90 days but firm conclusions could not be drawn from a limited number of studies. NIV weaning also reduced the duration of invasive ventilation and LOS in intensive care in COPD and the mixed ICU populations. Our findings are in agreement with contemporary literature in that the benefits of NIV weaning may stem from avoiding the injurious effects of prolonged invasive ventilation such as VAP, a complication that can often lengthen intensive care stay [51, 52]. It might also explain the survival benefit seen in COPD patients in whom such injurious effects may have the biggest impact. NIV has been used extensively to treat acute respiratory failure in patients with exacerbation of COPD. The beneficial effects of improved gas exchange reduce the work of respiratory muscles in respiratory failure and also facilitate resting of respiratory muscle, which may prove to be crucial to patient outcomes in this cohort [53]. In contrast to the previous Cochrane review, our results did not find an overall reduction in hospital LOS. As a result of insufficient data, our review was unable to draw any inferences on the impact of NIV weaning on weaning failure and health-related quality of life.
Bayesian methods have previously been used to perform meta-analyses for clinical trials to overcome the limitations of traditional meta-analysis, such as accounting for missing data, a small number of studies, sparse event data, uncertainty in the unknown model parameters, incorporating external information and handling complex models (such as those which include covariates) [54, 55]. We employed the Bayesian approach for its flexibility and ability to model a small number of studies and account for uncertainty in model parameters.
The main limitation of our systematic review is the variable quality of included studies. As most of the studies were not on a clinical trials register and did not have published protocols, it was not possible to assess reporting bias. There was often a lack of detailed description of the intervention and process of weaning and heterogeneity between studies in the patients included. Other valuable information such as sedation guidelines and weaning protocols were not available. Inconsistencies and variation in the reporting of adverse events precluded further analyses of other complications including weaning failure in the patient cohort. The need for core outcome sets in mechanical ventilation studies has been addressed by Core Outcomes in Ventilation Trials (COVenT) but the lack of consistent use of defined adverse events will continue to impact on the ability of future systematic reviews to reliably pool adverse event data [56, 57].
Despite evidence that critical care survivors can suffer from poor physical and psychological outcomes and long-term impact on quality of life [58, 59], only one study reported on long-term outcomes [13]. The study did not find any significant difference in the patient-centred outcome of health-related quality of life at 90 and 180 days but was not powered to do so [13]. Prevention of long-term sequelae and supporting patients post critical care discharge remains a challenge and it is important that future critical care research examines patient-centred long-term outcomes [60].
It remains difficult to combine the results of nine studies that used a pulmonary infective control (PIC) window instead of an SBT to determine whether the patients are ready to be extubated. The PIC window uses clinical signs to determine whether the patient has recovered from pulmonary infection after receiving invasive ventilation and adequate antibiotics for 6–7 days. These include a significant decrease in infectious infiltrations demonstrated by chest radiograph; significantly decreased quantity and viscosity of sputum; normal temperature and normalising of leukocyte count. Whilst popular in China, the ability to breathe spontaneously with reduced ventilator support is not tested and the clinical efficacy of the PIC window as an assessment of readiness to wean remains unproven [61]. Results from these studies may not be generalisable to intensive care populations in other countries.
Conclusions
Our review demonstrates that the use of NIV in weaning from mechanical ventilation, compared with ongoing invasive ventilation, reduces hospital mortality, the incidence of VAP and length of ICU stay, particularly in patients with COPD. On this basis, extubation to NIV may be a reasonable clinical strategy in patients that fail an SBT, particularly in patients with COPD.
References
ICNARC (2016) Key statistics from the Case Mix Programme — adult, general critical care units. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports/Summary-Statistics. Accessed 29 Sept 2018
Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P (2002) Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 287:345–355
Jeong BH, Ko MG, Nam J, Yoo H, Chung CR, Suh GY, Jeon K (2015) Differences in clinical outcomes according to weaning classifications in medical intensive care units. PLoS One 10:e0122810
Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, Burns SM, Epstein SK, Esteban A, Fan E, Ferrer M, Fraser GL, Gong MN, Hough CL, Mehta S, Nanchal R, Patel S, Pawlik AJ, Schweickert WD, Sessler CN, Strom T, Wilson KC, Morris PE (2017) An Official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: liberation from mechanical ventilation in critically ill adults. rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am J Respir Crit Care Med 195:120–133
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Landsberg JW (2018) Chapter 19e—Liberation from mechanical ventilation. In: Landsberg JW (ed) Clinical practice manual for pulmonary and critical care medicine. Elsevier, Philadelphia, pp e1–e13
Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA (2001) Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest 120:1262–1270
Béduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, Grelon F, Runge I, Terzi N, Grangé S, Barberet G, Guitard P-G, Frat J-P, Constan A, Chretien J-M, Mancebo J, Mercat A, Richard J-CM, Brochard L (2017) Epidemiology of weaning outcome according to a new definition. WIND Study 195:772–783
Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ (2017) Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004104.pub4
Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C, González M, Hill NS, Nava S, D’Empaire G, Anzueto A (2006) Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 130:1664–1671
Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, Navalesi P, Antonelli M, Brozek J, Conti G, Ferrer M, Guntupalli K, Jaber S, Keenan S, Mancebo J, Mehta S, Raoof S (2017) Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 50(2):1602426. https://doi.org/10.1183/13993003.02426-2016
Burns KE, Adhikari NK, Keenan SP, Meade MO (2010) Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004127.pub2
Perkins GD, Mistry D, Gates S, et al. (2018) Effect of protocolized weaning with early extubation to noninvasive ventilation vs invasive weaning on time to liberation from mechanical ventilation among patients with respiratory failure the breathe randomized clinical Trial. JAMA. https://doi.org/10.1001/jama.2018.13763
Gelman ACJ, Stern HS, Rubin DB (2003) Bayesian data analysis. Chapman and Hall/CRC, Boca Raton
Lv H (1981) Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat 6:107–128
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472
Burns KE, Adhikari NK, Meade MO (2003) Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev (4):CD004127
Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, Cook DJ, Ayas N, Adhikari NK, Hand L, Scales DC, Pagnotta R, Lazosky L, Rocker G, Dial S, Laupland K, Sanders K, Dodek P (2011) Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 183:E195–E214
Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G (1999) Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 160:86–92
Chen J, Qiu D, Tao D (2001) Time for extubation and sequential noninvasive mechanical ventilation in COPD patients with exacerbated respiratory failure who received invasive ventilation. Zhonghua Jie He He Hu Xi Za Zhi 24:99–100
Wang X, Du X, Zhang W (2004) Observation of the results and discussion on the timing of transition from invasive mechanical ventilation to noninvasive ventilation in COPD patients with concomitant acute respiratory failure. Shandong Med J 44:4–6
Wang C, Zhan QY, Cao ZX, Wei LQ, Cheng ZZ, Liu S, Zhang JI, Chen RC, Luo Q, Niu SF, Zhu L, Wu DW, Fang BM, Wu TH, Wang CZ, Ablinimit A, Liu YN (2005) Pulmonary infection control window in treatment of severe respiratory failure of chronic obstructive pulmonary diseases: a prospective, randomized controlled, multi-centred study. Chin Med J 118:1589–1594
Liu L, Qiu HB, Zheng RQ, Yang Y (2005) Prospective randomized controlled clinical study of early use of noninvasive positive pressure ventilation in the treatment for acute exacerbation of chronic obstructive pulmonary disease. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 17:477–480
Zou SH, Zhou R, Chen P, Luo H, Xiang XD, Lu YD, Zhu LY (2006) Application of sequential noninvasive following invasive mechanical ventilation in COPD patients with severe respiratory failure by investigating the appearance of pulmonary-infection-control-window. Zhong Nan Da Xue Xue Bao Yi Xue Ban 31:120–124
Trevisan CE, Vieira SR, Research Group in Mechanical Ventilation Weaning (2008) Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 12:R51
Charra B, Hachimi A, Benslama A, Motaouakkil S (2009) Contribution of noninvasive ventilation in the precocious extubation in the medical ICU. Crit Care 13:S5–S6
Girault C, Bubenheim M, Abroug F, Diehl JL, Elatrous S, Beuret P, Richecoeur J, L’Her E, Hilbert G, Capellier G, Rabbat A, Besbes M, Guérin C, Guiot P, Bénichou J, Bonmarchand G, VENISE Trial Group (2011) Noninvasive ventilation and weaning in patients with chronic hypercapnic respiratory failure: a randomized multicenter trial. Am J Respir Crit Care Med 184:672–679
Mohamed AI (2012) Elective early noninvasive ventilation as a weaning method of COPD patients. Eur Respir J 40(Suppl 56):P2041
Vaschetto R, Turucz E, Dellapiazza F, Guido S, Colombo D, Cammarota G, Della Corte F, Antonelli M, Navalesi P (2012) Noninvasive ventilation after early extubation in patients recovering from hypoxemic acute respiratory failure: a single-centre feasibility study. Intensive Care Med 38:1599–1606
Rong F (2012) Application of treating chronic obstructive pulmonary disease patients with respiratory failure with the sequential noninvasive and invasive ventilation. J Bengbu Med Coll 37:442–444
Carron M, Rossi S, Carollo C, Ori C (2014) Comparison of invasive and noninvasive positive pressure ventilation delivered by means of a helmet for weaning of patients from mechanical ventilation. J Crit Care 29:580–585
Mishra M, Chaudhri S, Tripathi V, Verma AK, Sampath A, Chauhan NK (2014) Weaning of mechanically ventilated chronic obstructive pulmonary disease patients by using non-invasive positive pressure ventilation: a prospective study. Lung India 31:127–133
Wang X, Xu S, Liu G, Caikai S (2014) Study of timing of invasive and noninvasive sequential ventilation in patients with acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 26:330–334
Tawfeek ME, Elnabtity AMA (2012) Noninvasive proportional assist ventilation may be useful in weaning patients who failed spontaneous breathing trial. Egypt J Anaesth 28:89–94
Guo F, Xu S, Liu G, Wang X (2015) An investigation of the efficacy of invasive-noninvasive sequential mechanical ventilation in senile patients with severe community-acquired pneumonia. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 27:595–600
Prasad SB, Chaudhry D, Khanna R (2009) Role of noninvasive ventilation in weaning from mechanical ventilation in patients of chronic obstructive pulmonary disease: an Indian experience. Indian J Crit Care Med 13:207–212
El-Shimy WS, Barima MA, El-Magd GHA, Mansour SA (2013) Non invasive ventilation versus synchronized intermittent mandatory ventilation with pressure support in weaning of COPD patients: comparative study. Egypt J Chest Dis Tuberc 62:159–166
Ferrer M, Esquinas A, Arancibia F, Bauer TT, Gonzalez G, Carrillo A, Rodriguez-Roisin R, Torres A (2003) Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 168:70–76
Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, Brigada P, Fracchia C, Rubini F (1998) Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 128:721–728
Trevisan CE, Vieira SR, Research Group in Mechanical Ventilation Weaning (2008) Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 12:R51
Chaudhri S, Mishra M, Verma A, Sampath A, Rai O (2009) Utility of non invasive pressure ventilation (NIPPV) for weaning COPD patients from invasive mechanical ventilation (IMV). In: European Respiratory Society Annual Congress, Vienna, Austria, 12–16 September 2009
Laiq N, Khan RA, Malik A (2013) Non-invasive positive pressure ventilation facilitates early extubation in post operative cardiac patients. J Postgrad Med Inst 27(4):361–365
Matic I, Sakic-Zdravcevic K, Jurjevic M (2007) Comparison of invasive and noninvasive mechanical ventilation for patients with chronic obstructive pulmonary disease: randomized prospective study. Period Biologorum 109:137–145
Burns KE, Meade MO, Premji A, Adhikari NK (2014) Noninvasive ventilation as a weaning strategy for mechanical ventilation in adults with respiratory failure: a Cochrane systematic review. CMAJ 186:E112–E122
Funk G-C, Bauer P, Burghuber OC, Fazekas A, Hartl S, Hochrieser H, Schmutz R, Metnitz P (2013) Prevalence and prognosis of COPD in critically ill patients between 1998 and 2008. Eur Respir J 41(4):792–799. https://doi.org/10.1183/09031936.00226411
Vincent J-L, Marshall JC, Ñamendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, Jimenez E, Sakr Y (2014) Assessment of the worldwide burden of critical illness: the Intensive Care Over Nations (ICON) audit. Lancet Respir Med 2:380–386
Rello J, Rodriguez A, Torres A, Roig J, Sole-Violan J, Garnacho-Montero J, de la Torre MV, Sirvent JM, Bodi M (2006) Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J 27(6):1210–1216. https://doi.org/10.1183/09031936.06.00139305
Mayr VD, Dünser MW, Greil V, Jochberger S, Luckner G, Ulmer H, Friesenecker BE, Takala J, Hasibeder WR (2006) Causes of death and determinants of outcome in critically ill patients. Crit Care 10(6):R154
Orban J-C, Walrave Y, Mongardon N, Allaouchiche B, Argaud L, Aubrun F, Barjon G, Constantin J-M, Dhonneur G, Durand-Gasselin J, Dupont H, Genestal M, Goguey C, Goutorbe P, Guidet B, Hyvernat H, Jaber S, Lefrant J-Y, Mallédant Y, Morel J, Ouattara A, Pichon N, Guérin Robardey A-M, Sirodot M, Theissen A, Wiramus S, Zieleskiewicz L, Leone M, Ichai C (2017) Causes and characteristics of death in intensive care units: a prospective multicenter study. Anesthesiology 126:882–889
Blackwood B, Alderdice F, Burns K, Cardwell C, Lavery G, O’Halloran P (2011) Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. BMJ 342:c7237. https://doi.org/10.1136/bmj.c7237
Langer M, Cigada M, Mandelli M, Mosconi P, Tognoni G (1987) Early onset pneumonia: a multicenter study in intensive care units. Intensive Care Med 13:342–346
Chastre J, Fagon J-Y (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Lightowler JV, Wedzicha JA, Elliott MW, Ram FSF (2003) Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 326:185
Ibrahim JG, Chen M-H, Chu H (2012) Bayesian methods in clinical trials: a Bayesian analysis of ECOG trials E1684 and E1690. BMC Med Res Methodol 12:183
Babapulle MN, Joseph L, Bélisle P, Brophy JM, Eisenberg MJ (2004) A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet 364:583–591
Blackwood B, Ringrow S, Clarke M, Marshall J, Rose L, Williamson P, McAuley D (2015) Core Outcomes in Ventilation Trials (COVenT): protocol for a core outcome set using a Delphi survey with a nested randomised trial and observational cohort study. Trials 16:368
Ringrow S, McAuley D, Clarke M, Marshall J, Connolly B, Rose L, Blackwood B (2017) S133 A core outcome set for mechanical ventilation trials: the covent study. Thorax 72:A79–A80
Hatch R, Young D, Barber V, Harrison DA, Watkinson P (2017) The effect of postal questionnaire burden on response rate and answer patterns following admission to intensive care: a randomised controlled trial. BMC Med Res Methodol 17:49
Griffiths JA, Morgan K, Barber VS, Young JD (2008) Study protocol: the Intensive Care Outcome Network (‘ICON’) study. BMC Health Serv Res 8:132
Reay H, Arulkumaran N, Brett SJ (2014) Priorities for future intensive care research in the UK: results of a James Lind Alliance Priority Setting Partnership. J Intensive Care Soc 15:288–296
Lv Y, Lv Q, Lv Q, Lai T (2017) Pulmonary infection control window as a switching point for sequential ventilation in the treatment of COPD patients: a meta-analysis. Int J Chron Obstruct Pulmon Dis 12:1255–1267
Acknowledgements
JY and KC are supported as National Institute of Health Research post-doctoral research fellows. GDP and SG are supported as NIHR Senior Investigators. GDP is a Director of Research for the Intensive Care Foundation. This review has been supported by the NIHR Health Technology Assessment Programme (HTA 10/134).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
GDP was chief investigator of the Breathe trial. NH and SG were Breathe trial co-investigators. JY and KC are supported by NIHR Post-Doctoral Fellowships. EGR is supported by MRC methodology grant (Grant number: MR/N028287/1).
Additional information
Systematic review registration: PROSPERO (CRD42017076522).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yeung, J., Couper, K., Ryan, E.G. et al. Non-invasive ventilation as a strategy for weaning from invasive mechanical ventilation: a systematic review and Bayesian meta-analysis. Intensive Care Med 44, 2192–2204 (2018). https://doi.org/10.1007/s00134-018-5434-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5434-z