Abstract
Purpose
Whether closed tracheal suctioning systems (CTSS) reduce the incidence of ventilator-associated pneumonia (VAP) compared with open tracheal suctioning systems (OTSS) is inconclusive. We conducted a systematic review and meta-analysis of randomized controlled trials that compared CTSS and OTSS.
Methods
PubMed, the Cochrane Central Register of Controlled Trials, the Web of Science, Google Scholar, and a clinical trial registry from inception to October 2014 were searched without language restrictions. Randomized controlled trials of CTSS and OTSS that compared VAP in mechanically ventilated adult patients were included. The primary outcome was the incidence of VAP. Secondary outcomes were mortality and length of mechanical ventilation. Data were pooled using the random effects model.
Results
Sixteen trials with 1,929 participants were included. Compared with OTSS, CTSS was associated with a reduced incidence of VAP (RR 0.69; 95 % CI 0.54–0.87; Q = 26.14; I 2 = 46.4 %). Compared with OTSS, CTSS was not associated with reduction of mortality (RR 0.96; 95 % CI 0.83–1.12; Q = 2.27; I 2 = 0.0 %) or reduced length of mechanical ventilation (WMD −0.45 days; 95 % CI −1.25 to 0.36; Q = 6.37; I 2 = 5.8 %). Trial sequential analysis suggested a lack of firm evidence for 20 % RR reduction in the incidence of VAP. The limitations of this review included underreporting and low quality of the included trials, as well as variations in study procedures and characteristics.
Conclusions
Based on current, albeit limited evidence, it is unlikely that CTSS is inferior to OTSS regarding VAP prevention; however, further trials at low risk of bias are needed to confirm or refute this finding.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is one of the common nosocomial infections in intensive care units (ICUs). It is reported that 6–52 % of mechanically ventilated patients develop VAP [1–4]. VAP is associated with prolonged ICU and hospital stays [5, 6] and mortality [7, 8]. The annual cost for VAP is considerable and approximated $3.0 billion USD [9]. Thus, the prevention of VAP has substantial merits from the clinical and societal perspectives.
Currently, two types of endotracheal suctioning systems are available: closed tracheal suction systems (CTSS) and open tracheal suction systems (OTSS). CTSS allow multiple episodes of endotracheal suctioning without disconnecting the patient from the ventilator. Their suggested advantages compared with OTSS use include limited environmental and personnel contamination [10], maintained positive end expiratory pressure, lung volume, and oxygenation [11, 12], and fewer physiologic disturbances during suctioning such as decreased arterial deoxygenation, increased heart rate, and increased mean arterial pressure [13, 14].
Another potential advantage of CTSS use is the prevention of VAP. Previous systematic reviews and meta-analyses have concluded that CTSS use has no benefit over OTSS use in preventing VAP [13, 15–20]. However, they were based on a relatively small number of trials, and some even suggested a potential publication bias [13, 20]. Trials published in some non-English languages were not included.
Therefore, we conducted a systematic review and meta-analysis to reassess the efficacy of CTSS use to prevent VAP in mechanically ventilated adult patients, in comparison with OTSS use. Recent trials and trials published in non-English languages were included, and trial sequential analysis was conducted to challenge the robustness of the available evidence.
Materials and methods
Study selection
The study protocol was pre-specified as a protocol and followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) Statement for reporting on systematic reviews [21]. We searched PubMed, the Cochrane Central Register of Controlled Trials, and the Web of Science. The search strategy was listed in the protocol (Supplementary File). Clinicaltrial.gov, Google Scholar, and the references of retrieved articles and previous systematic reviews were reviewed for potentially relevant trials. No language restrictions were placed on the search. The last search was conducted on October 27, 2014.
Eligibility criteria
Randomized controlled trials that compared CTSS and OTSS use in adult patients (aged 18 years or over) on mechanical ventilation in ICUs were included. Trials published in abstracts were also included if pertinent data on patients’ characteristics and outcomes of interest were available. Crossover trials and trials conducted on neonates and infants were excluded.
Data abstraction and risk of bias assessment
At least two of the reviewers (A.K., N.U., and J.F.) independently extracted the following information in duplicate: (1) study characteristics (the types of ICUs, sample size, inclusion or exclusion of patients with pneumonia at admission to ICUs, and definitions of VAP); (2) participants’ demographics (age and sex); (3) interventions (CTSS brand names, the cycle of CTSS exchange, industry sponsorship); and (4) outcomes of interest. Attempts were made to contact the original authors for more details. The authors were considered unresponsive, when three e-mails were sent and no reply was obtained. At least two of the authors (A.K., N.U., and J.F.) independently assessed the risk of bias, using the Risk of Bias tool recommended by the Cochrane Collaborations [22], as well as the sponsorship. Any disagreement was resolved through discussion.
Statistical analysis
The primary outcome was the incidence of VAP. Any definitions of VAP were allowed. Secondary outcomes were mortality and length of mechanical ventilation. When trials had more than one arm for the intervention, the data were pooled into a single group [22]. When trials had zero events in either arm, continuity corrections were applied with addition of 0.5 to each cell of 2 × 2 tables from the trial [23]. Dichotomous outcomes were combined and presented as risk ratios (RRs) with associated 95 % confidence intervals (CIs). Length of mechanical ventilation was combined using weighted mean differences (WMD). We a priori knew that the included populations would be clinically heterogenous, and thus data were pooled using the DerSimonian and Laird random-effects model [24]. Statistical heterogeneity was assessed with the I 2 and Q statistics [25]. When significant heterogeneity was identified (I 2 ≥ 50 % or p < 0.1), meta-regression analysis was conducted to investigate the potential sources of heterogeneity. We performed subgroup analysis stratified by the ICU population, as done in the Cochrane review [19] and on our hypothesis that there is no difference in the effect size among subgroups. A subgroup analysis stratified by the cycle of exchanging CTSS and meta-regression analysis to examine the relationship between the cycle and the effect size were also conducted. The test-of-interaction was performed in subgroup analyses [26]. Sensitivity analyses were conducted by excluding trials that included patients with pneumonia at admission, and by excluding trials with unclear or high risk of bias in any domain of sequence generation, allocation concealment, or blinding of outcome assessors. Publication bias was tested using Egger’s method [27]. All these analyses were conducted with Stata v.11.2 (Stata, College Station, TX, USA).
A meta-analysis may suffer from the type I error due to an increased risk of random error due to sparse data, and due to repeated significance testing when meta-analyses are updated with new trials [28]. Sensitivity analysis with trial sequential analysis (TSA) on our primary outcome (VAP) was additionally performed to adjust for random error and repetitive testing. Meta-analysis monitoring boundaries and required information size (cumulated sample size of included trials) were calculated, with D2 (diversity adjusted information size) and adjusted 95 % CIs. The idea behind TSA is that if the cumulative Z-curve crosses the boundary, a sufficient level of evidence is reached and no further trials are needed. If the Z-curve does not cross the boundary and the required information size is not been reached, evidence is insufficient to allow investigators to conduct further trials for a conclusion. We conducted TSA to maintain a type I error of 5 %, and calculated the required information size with an anticipated intervention size of 20 % relative risk reduction (RRR), at a power of 80 % [29]. We conducted TSA with TSA 0.9 (The Copenhagen Trial Unit, Copenhagen, Denmark).
Results
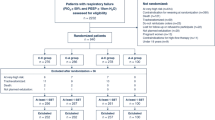
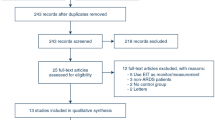
The search produced 400 articles (Supplementary Fig. 1). After application of inclusion and exclusion criteria, 16 randomized, controlled trials that compared CTSS and OTSS were identified [14, 30–44]. Two major CTSS sales companies were contacted, but no new information was obtained. A total of 1,929 mechanically-ventilated patients was included in the analysis (Table 1). The mean age of participants was 48.3 years, and 29 % was women. The median sample size was 74. The follow-up periods were reported in six trials, and ranged from 7 to 31 days [30, 33, 34, 37, 41, 44]. Four trials were conducted in medical ICUs [32, 35, 36, 40], four in surgical ICUs [14, 30, 31, 38], and six in mixed (medico-surgical) ICUs [33, 34, 37, 39, 43, 44]; the remainder was unclear. Six trials used Trach Care, two Steri Cath, two Hi Care, and one Ty Care as the CTSS; the CTSS brand was unclear in five trials. The cycle of CTSS exchange varied considerably; nine trials exchanged CTSS every 24 h [14, 30, 31, 34, 35, 38, 40, 42, 44], one every 72 h [37], one every 168 h [32], and one trial included groups that exchanged CTSS every 24 and 48 h [43], respectively. All trials except two excluded patients with pneumonia at the onset of the studies [32, 41]. Fourteen trials set VAP as the primary outcome [14, 31–40, 42–44], four of which a priori calculated the sample size [32–34, 37]. Twelve trials were reported in English, two in Chinese [43, 44], one in Korean [37], and one in Arabic [42].
Study quality
Overall, four trials (25 %) had adequate sequence generation, whereas two (13 %) had adequate concealed allocation (Supplementary Table 1). Outcome assessors were judged to be adequately blinded in four trials (25 %). Only one trial was assessed as overall low risk of bias [32]. Two studies (13 %) disclosed the involvement of industry sponsorship; CTSS was provided for one trial, and a support grant was given for the other trial.
Primary outcome analysis
Use of CTSS was associated with a reduced incidence of VAP compared with OTSS (RR 0.69; 95 % CI 0.54–0.87; Q = 26.14; df = 14; I 2 = 46.4 %, p = 0.03) (Fig. 1). The point estimates of the effect size were similar across the subgroups (test-of-interaction p = 0.95) (Table 2). No publication bias was evident (p = 0.13). The pooled data had moderate heterogeneity. Meta-regression analysis showed that sample size (p = 0.06), age (p = 0.35), sex (p = 0.50), publication date (p = 0.53), publication type (p = 0.65), single- or multicenter study (p = 0.75) and any risk of bias were not the source of the heterogeneity. Compared to OTSS use, the use of CTSS was not associated a reduced incidence of VAP in the only one trial with overall low risk of bias (RR 0.56; 95 % CI 0.27–1.14), whereas pooled results from the trials with overall unclear or high risk of bias showed beneficial effect (RR 0.70; 95 % CI 0.54–0.90; Q = 25.51; df = 13; I 2 = 49.0 %, p = 0.02).
Secondary outcome analysis
Compared with use of OTSS, use of CTSS was not associated with reduction of mortality (RR 0.96; 95 % CI 0.83–1.12; Q = 2.27; df = 6; I 2 = 0.0 %; p = 0.89) (Supplementary Fig. 2) or a shorter length of mechanical ventilation (WMD −0.45 days; 95 % CI −1.25 to 0.36; Q = 6.37; df = 6; I 2 = 5.8 %; p = 0.38) (Supplementary Fig. 3). No publication bias was evident in mortality (p = 0.94) or length of mechanical ventilation (p = 0.90).
Subgroup analysis stratified by the cycle of CTSS exchange
An analysis of each outcome stratified by the cycle of CTSS exchange was conducted (Table 3). Use of CTSS was associated with a reduced incidence of VAP in the subgroup with 24-h and 72-h cycles, and reduced length of mechanical ventilation in the subgroup with a 48-h cycle. No significant differences were found in the other subgroups. Meta-regression analyses showed that there was no relationship between the cycle of CTSS exchange and VAP (p = 0.50), mortality (p = 0.33), or length of mechanical ventilation (p = 0.78).
Sensitivity analyses
Of the 16 trials, 2 trials included 252 patients with pneumonia at the initiation of the studies [32, 41]. When these two trials were excluded, use of CTSS was associated with a reduced incidence of VAP (RR 0.71; 95 % CI 0.54–0.94; Q = 24.08; df = 12; I 2 = 50.2 %; p = 0.02), but it was not associated with reduced mortality (RR 1.03; 95 % CI 0.86–1.23) or shorter length of mechanical ventilation (WMD −0.47 days; 95 % CI −1.43 to 0.50). Meta-regression analysis failed to find the source of heterogeneity in the pooled outcome of VAP.
We additionally conducted sensitivity analyses on all outcomes (Supplementary Table 2). Use of CTSS tended to lower the incidence of VAP in all sensitivity analyses, but the statistical significance disappeared due to a limited number of trials and less statistical power. The results of sensitivity analyses on mortality and the length of mechanical ventilation were consistent with the secondary outcome analysis.
We reanalyzed the data using TSA. The required diversity (D 2 = 53 %, model variance-based) adjusted information size of 4331 participants was calculated, based on 26 % events in the control group, a type I error of 5 %, a power of 80 %, and an RRR of 20 %. The cumulative Z-curve did not cross the trial sequential monitoring boundary for benefit, or the required information size was not reached (Fig. 2). This implies the lack of evidence of CTSS use on the incidence of VAP.
Discussion
Our traditional meta-analysis showed that use of CTSS was associated with a 30 % reduction in VAP development, compared with use of OTSS. While each subgroup by the type of ICU included a small number of trials, and thus had a wide confidential interval and moderate heterogeneity, the point estimate of the effect size was similar across the subgroups. There was no significant difference in mortality or length of mechanical ventilation between CTSS and OTSS use. Use of CTSS tended to lower the incidence of VAP, but the statistical significance disappeared owing to less statistical power due to a limited number of trials included in the sensitivity analyses. Trials with high risk of bias might have overestimated the intervention effect of CTSS in the traditional meta-analysis, and thus the results should be interpreted cautiously. TSA as sensitivity analysis also revealed that the evidence is lacking to suggest that use of CTSS is associated with a lower incidence of VAP, and further research is needed.
Exogenous bacteria travel to the lower respiratory tract in mechanically ventilated patients, from the oropharynx along the external surface of the tube to the trachea, or by inadvertent cross-contamination during the disconnection of the respiratory circuit for suction, and they may result in VAP development [35]. Endotracheal tubes with subglottic secretion might block the former route [45]. Use of CTSS might reduce the chance of the latter mechanism, thereby reducing VAP development. However, prior evidence has been inconclusive with respect to this hypothesis.
Previous systematic reviews and meta-analyses of randomized trials have concluded that use of CTSS was not associated with a reduced incidence of VAP compared with use of OTSS [13, 15–20]. Some also suggested a potential publication bias. The present traditional meta-analysis showed that the incidence of VAP was significantly reduced by CTSS use. The differences in the findings between previous reviews and the present review might be attributed to two factors: (1) the number of included trials was greater, and (2) all newly included trials favored CTSS use over OTSS use. There was no evidence of publication bias in the present analysis. However, TSA indicated that the current evidence as to whether the use of CTSS is superior to the use of OTSS in preventing VAP was still lacking.
Earlier guidelines for the prevention of VAP were inconclusive about the effectiveness of CTSS as a VAP prevention measure [46, 47]. Some guidelines have favored CTSS over OTSS use for cost and safety considerations, despite the scarcity of favorable evidence supporting CTSS use for the prevention of VAP [48–51]. The present analysis showed that the number needed to prevent (NNP) with CTSS for reducing one incidence of VAP was 8.85 (95 % CI 5.62–21.27). However, scarcity of high-quality trials as well as a lack of firm beneficial evidence on CTSS rendered this issue inconclusive.
Our study triggered uncertainty regarding the optimal timing of CTSS exchange. Trials included in the present review exchanged the CTSS at 24, 48, 72, and 168 h, respectively. Meta-regression analysis suggested that there was no relationship between the cycle and the incidence of VAP. One randomized trial suggested that the incidence of VAP was not different between the 24-h and 48-h cycles of CTSS exchange, but it was underpowered [52]. Another randomized trial compared daily and no routine exchange of in-line catheters of CTSS [53]. It suggested that, while the incidence of VAP and hospital mortality were similar for the two methods, no routine exchange of in-line catheters reduced the cost. Currently, most manufacturers recommend daily CTSS exchange, but no direct evidence supports this. Sufficiently powered trials are needed to determine the optimal cycle of CTSS exchange. A cost-effectiveness analysis concerning the cycle of CTSS exchange, incidence of VAP, and related costs should subsequently be considered.
Our study has some strengths. First, a comprehensive search for trials was conducted. During the search, three Chinese trials that favored CTSS use as a prevention measure against nosocomial pneumonia were not included, because the diagnostic criteria for nosocomial pneumonia in mechanically ventilated patients were unclear. Nevertheless, four trials reported in non-English languages were included, and the total number of trials was the largest to date. Second, appropriate and relevant subgroup and meta-regression analyses were conducted due to the large number of trials. This made the analysis more rigorous. Third, the study protocol was preplanned, as recommended by the Cochrane Collaboration [22]. Finally, the introduction of TSA as well as sensitivity analyses using the traditional meta-analytic methods led to our cautious interpretation of the current evidence about CTSS, which otherwise supported the use of CTSS as a preventive measure against VAP.
The present study has limitations. First, patients’ characteristics, study protocols including prophylactic antibiotics and oral care, and the risk of VAP were not uniform across trials. The pooled analysis of VAP showed significant heterogeneity in the subgroup of mixed ICUs. However, a lack of details about these factors precluded investigating this heterogeneity. Second, many trials underreported their methodology. Of the 16 trials, 13 were published after 1996, when the CONSORT (Consolidated Standards of Reporting Trials) statement was developed to enhance researchers’ complete, clear, and transparent reporting of randomized trials [54]. More than half of these trials lacked information on sequence generation, allocation concealment, and blinding of outcome assessors. Meta-regression analysis showed that these risks of bias did not affect the pooled outcomes. However, high-quality trials are still warranted. Third, most trials included in our analysis was relatively small. It is known that most large treatment effects emerge from small-sized trials [55], while meta-regression analysis in our study barely failed to show that smaller trials tended to show favorable effectiveness of CTSS (p = 0.06). Thus, when future trials are conducted with larger sample sizes, the results may not support our study.
Conclusion
Our traditional meta-analysis suggested that CTSS use was associated with a reduced incidence of VAP compared with OTSS use. CTSS use showed no differences in terms of mortality and length of mechanical ventilation compared with OTSS use. However, sensitivity analyses including TSA suggested the scarcity of high-quality trials and the lack of firm evidence for the benefit of CTSS use compared with OTSS use in reducing VAP. This does not yet support the use of CTSS as a VAP prevention measure, which was advocated in some current guidelines. High-quality trials with a better reporting of trial results are still needed.
References
Apostolopoulou E, Bakakos P, Katostaras T, Gregorakos L (2003) Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens, Greece. Respir Care 48:681–688
Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, Dudeck MA, Pollock DA, Horan TC (2009) National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 37:783–805
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Rello J, Rue M, Jubert P, Muses G, Sonora R, Valles J, Niederman MS (1997) Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med 25:1862–1867
Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C (1999) The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med 159:1249–1256
Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH, Group VAPOSA (2002) Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 122:2115–2121
Melsen WG, Rovers MM, Bonten MJ (2009) Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med 37:2709–2718
Safdar N, Dezfulian C, Collard HR, Saint S (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 33:2184–2193
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW (2013) Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Int Med 173:2039–2046
Cobley M, Atkins M, Jones PL (1991) Environmental contamination during tracheal suction. A comparison of disposable conventional catheters with a multiple-use closed system device. Anaesthesia 46:957–961
Cereda M, Villa F, Colombo E, Greco G, Nacoti M, Pesenti A (2001) Closed system endotracheal suctioning maintains lung volume during volume-controlled mechanical ventilation. Intensive Care Med 27:648–654
Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, Richard JC, Mancebo J, Lemaire F, Brochard L (2003) Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med 167:1215–1224
Jongerden IP, Rovers MM, Grypdonck MH, Bonten MJ (2007) Open and closed endotracheal suction systems in mechanically ventilated intensive care patients: a meta-analysis. Crit Care Med 35:260–270
Johnson KL, Kearney PA, Johnson SB, Niblett JB, MacMillan NL, McClain RE (1994) Closed versus open endotracheal suctioning: costs and physiologic consequences. Crit Care Med 22:658–666
Niel-Weise BS, Snoeren RL, van den Broek PJ (2007) Policies for endotracheal suctioning of patients receiving mechanical ventilation: a systematic review of randomized controlled trials. Infect Control Hosp Epidemiol 28:531–536
Overend TJ, Anderson CM, Brooks D, Cicutto L, Keim M, McAuslan D, Nonoyama M (2009) Updating the evidence-base for suctioning adult patients: a systematic review. Can Respir J 16:e6–e17
Peter JV, Chacko B, Moran JL (2007) Comparison of closed endotracheal suction versus open endotracheal suction in the development of ventilator-associated pneumonia in intensive care patients: an evaluation using meta-analytic techniques. Indian J Med Sci 61:201–211
Siempos II, Vardakas KZ, Falagas ME (2008) Closed tracheal suction systems for prevention of ventilator-associated pneumonia. Br J Anaesth 100:299–306
Subirana M, Sola I, Benito S, (2007) Closed tracheal suction systems versus open tracheal suction systems for mechanically ventilated adult patients. The Cochrane database of systematic reviews: CD004581
Vonberg RP, Eckmanns T, Welte T, Gastmeier P (2006) Impact of the suctioning system (open vs. closed) on the incidence of ventilation-associated pneumonia: meta-analysis of randomized controlled trials. Intensive Care Med 32:1329–1335
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Higgins J, Green S (2008) Cochrane handbook for systematic reviews of interventions. Wiley, Chichester
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23:1351–1375
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326:219
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Brok J, Thorlund K, Gluud C, Wetterslev J (2008) Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 61:763–769
Wetterslev J, Thorlund K, Brok J, Gluud C (2009) Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 9:86
Adams DH, Hughes M, Elliott TS (1997) Microbial colonization of closed-system suction catheters used in liver transplant patients. Intensive Crit Care Nurs 13:72–76
Combes P, Fauvage B, Oleyer C (2000) Nosocomial pneumonia in mechanically ventilated patients, a prospective randomised evaluation of the Stericath closed suctioning system. Intensive Care Med 26:878–882
David D, Samuel P, David T, Keshava SN, Irodi A, Peter JV (2011) An open-labelled randomized controlled trial comparing costs and clinical outcomes of open endotracheal suctioning with closed endotracheal suctioning in mechanically ventilated medical intensive care patients. J Crit Care 26:482–488
Lorente L, Lecuona M, Jimenez A, Mora ML, Sierra A (2006) Tracheal suction by closed system without daily change versus open system. Intensive Care Med 32:538–544
Lorente L, Lecuona M, Martin MM, Garcia C, Mora ML, Sierra A (2005) Ventilator-associated pneumonia using a closed versus an open tracheal suction system. Crit Care Med 33:115–119
Rabitsch W, Kostler WJ, Fiebiger W, Dielacher C, Losert H, Sherif C, Staudinger T, Seper E, Koller W, Daxbock F, Schuster E, Knobl P, Burgmann H, Frass M, (2004) Closed suctioning system reduces cross-contamination between bronchial system and gastric juices. Anesth Analg 99: 886-892, table of contents
Topeli A, Harmanci A, Cetinkaya Y, Akdeniz S, Unal S (2004) Comparison of the effect of closed versus open endotracheal suction systems on the development of ventilator-associated pneumonia. J Hosp Infect 58:14–19
Lee ES, Kim SH, Kim JS (2004) Effects of a closed endotracheal suction system on oxygen saturation, ventilator-associated pneumonia, and nursing efficacy. Taehan Kanho Hakhoe chi 34:1315–1325
Deppe SA, Kelly JW, Thoi LL, Chudy JH, Longfield RN, Ducey JP, Truwit CL, Antopol MR (1990) Incidence of colonization, nosocomial pneumonia, and mortality in critically ill patients using a Trach Care closed-suction system versus an open-suction system: prospective, randomized study. Crit Care Med 18:1389–1393
Zeitoun SS, de Barros AL, Diccini S (2003) A prospective, randomized study of ventilator-associated pneumonia in patients using a closed vs. open suction system. J Clin Nurs 12:484–489
Conrad SA, George RB, Romero MD, Owens MW (1989) Comparison of nosocomial pneumonia rates in closed and open tracheal suction systems. Chest 96:184S
Welte T, Ziesing S, Schulte S, Wagner TOF (1997) Incidence of ventilator associated pneumonia in mechanically ventilated patients: a comparison of closed versus open endotracheal suctioning. Eur Respir J 10(Suppl):319
Fakhar HRE, Rezaie K, Kohestani HR (2010) Effect of closed endotracheal suction on incidence of ventilator-associated pneumonia. Sci J Kurd Univ Med Sci 15:79–87
Wang L, Chao YG, Li LM, Bian WS, QG. J, (2006) Clinical observation on the prevention of VAP by the closed type tracheal suctioning. Shan Dong Yi Yao 46:50–51
Li JQ, Li XY, He J (2007) Influence of different ways of sputumn suctioning on ventilator-associated pneumonia. J Nurs Sci 12:20–21
Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK (2011) Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med 39:1985–1991
Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R, Cdc, Healthcare Infection Control Practices Advisory C, (2004) Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recommendations and reports: morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 53: 1–36
Torres A, Carlet J (2001) Ventilator-associated pneumonia. European Task Force on ventilator-associated pneumonia. Eur Respir J 17:1034–1045
American Association for Respiratory C (2010) AARC Clinical Practice Guidelines. Endotracheal suctioning of mechanically ventilated patients with artificial airways 2010. Respir Care 55:758–764
Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L, Muscedere J, Foster D, Mehta N, Hall R, Brun-Buisson C, Canadian Critical Care Trials G, Canadian Critical Care S (2004) Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med 141: 305-313
Hess DR, Kallstrom TJ, Mottram CD, Myers TR, Sorenson HM, Vines DL, American Association for Respiratory C (2003) Care of the ventilator circuit and its relation to ventilator-associated pneumonia. Respir Care 48:869–879
Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D, Committee VAPG, The Canadian Critical Care Trials G (2008) Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care 23:126–137
Darvas JA, Hawkins LG (2003) The closed tracheal suction catheter: 24 hour or 48 hour change? Aust Crit 16:86–92
Kollef MH, Prentice D, Shapiro SD, Fraser VJ, Silver P, Trovillion E, Weilitz P, von Harz B, St John R (1997) Mechanical ventilation with or without daily changes of in-line suction catheters. Am J Respir Crit Care Med 156:466–472
Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF (1996) Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 276:637–639
Pereira TV, Horwitz RI, Ioannidis JP (2012) Empirical evaluation of very large treatment effects of medical interventions. JAMA 308:1676–1684
Acknowledgments
The authors would like to thank Dr. Arzu Topeli and Dr. Deepu David for providing relevant information, and would like to thank Ms. Ryoko Ono for editing the figures.
Conflicts of interest
None to declare for any author.
Financial disclosures
None to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuriyama, A., Umakoshi, N., Fujinaga, J. et al. Impact of closed versus open tracheal suctioning systems for mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med 41, 402–411 (2015). https://doi.org/10.1007/s00134-014-3565-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3565-4