Abstract
Purpose
To analyze trends in incidence and mortality of candidemia in intensive care units (ICUs) vs. non-ICU hospitalized patients and to determine risk factors for infection by specific species and for death.
Methods
Active hospital-based surveillance program of incident episodes of candidemia due to common species in 24 tertiary care hospitals in the Paris area, France between October 2002 and September 2010.
Results
Among 2,507 adult cases included, 2,571 Candida isolates were collected and species were C. albicans (56 %), C. glabrata (18.6 %), C. parapsilosis (11.5 %), C. tropicalis (9.3 %), C. krusei (2.9 %), and C. kefyr (1.8 %). Candidemia occurred in ICU in 1,206 patients (48.1 %). When comparing ICU vs. non-ICU patients, the former had significantly more frequent surgery during the past 30 days, were more often preexposed to fluconazole and treated with echinocandin, and were less frequently infected with C. parapsilosis. Risk factors and age remained unchanged during the study period. A significant increased incidence in the overall population and ICU was found. The odds of being infected with a given species in ICU was influenced by risk factors and preexposure to fluconazole and caspofungin. Echinocandins initial therapy increased over time in ICU (4.6 % first year of study, to 48.5 % last year of study, p < 0.0001). ICU patients had a higher day-30 death rate than non-ICU patients (odds ratio [OR] 2.12; 95 % confidence interval [CI] 1.66–2.72; p < 0.0001). The day-30 and early (<day 8) death rates increased over time in ICU (from 41.5 % the first to 56.9 % the last year of study (p = 0.001) and 28.7–38.8 % (p = 0.0292), respectively). Independent risk factors for day-30 death in ICU were age, arterial catheter, Candida species, preexposure to caspofungin, and lack of antifungal therapy at the time of blood cultures results (p < 0.05).
Conclusions
The availability of new antifungals and the publication of numerous guidelines did not prevent an increase of candidemia and death in ICU patients in the Paris area.
Similar content being viewed by others
Introduction
Candidemia currently represents up to 5.6–10 % of nosocomial bloodstream infections [1–3] with associated mortality and increased length of stay and cost [4]. It is therefore a public health concern everywhere [5]. Intensive care units (ICU) are increasingly involved, with more empiric or preemptive antifungal strategies being implemented in patients deemed at risk [6–8]. Many changes have occurred over the last decade with the availability of echinocandins, the implementation of management guidelines [9, 10], and, at least in some centers, the extended availability of new tools with improved diagnostic performances. Therefore, it is of utmost importance to analyze whether all these (expensive) changes impacted the incidence of candidemia and its associated death.
The analysis of epidemiological trends mainly relies on hospital registers which are often focused on a specific population [11, 12], based on a single center [13], limited to a short period of time [14], or restricted to microbiological data with limited clinical documentation [1]. We launched in 2002 a large prospective hospital-based surveillance program for patients developing candidemia in the Paris area (France). Our expectation was that a large population of patients over a long period of time without selection bias related to the cause of hospitalization cause could unravel some epidemiological shifts, in particular by comparing populations of patients with different underlying diseases. The goals of the study were to describe adult patients with candidemia, to analyze trends in incidence and mortality of candidemia in ICU versus non-ICU hospitalized patients, and to determine risk factors for infection by specific species and risk factors for death.
Materials and methods
Population studied and isolates characterization
A sustainable active surveillance program on yeast fungemia (YEASTS program) was implemented in October 2002 with participation of 24 short-stay university hospitals in the Paris area. All blood cultures positive with yeasts were reported by the participants, with clinical and epidemiological data (age, recent surgery within 30 days, cancer, hematological malignancy, transplantation, HIV serostatus, immunosuppressive therapy, intravenous drug addiction, invasive procedures, and recent administration of antifungals 30 days prior to candidemia), filled on a standardized form, on a secured website and all isolates were sent to the French National Reference Center for Invasive Mycoses and Antifungals (NRCMA). There, isolates were checked for purity and identified to the species level using carbon assimilation profiles (ID32C, bioMérieux) or nucleotide sequencing as needed [15]. Because of their low proportion, C. orthopsilosis and C. metapsilosis were included with C. parapsilosis in a “C. parapsilosis” complex. The current analysis concerns the incident episodes of candidemia due to common species (i.e., those accounting for at least 1.5 % of all Candida spp.) recorded in adults patients (at least 15 years old) between October 2002 and September 2010.
Definitions
The date of candidemia was the day of blood sampling (day 0). An incident case corresponded to the first episode of positive blood culture. A recurrent episode was considered in case of isolation of the same species at least 10 days after the initial isolation or of a new species with no time limit. To avoid autocorrelation, only incident episodes were considered in the subsequent analysis. Both single (one Candida species) and mixed (more than one Candida species) infections were considered. ICU patients were those hospitalized in ICU at the time of sampling and we individualized candidemia episodes occurring within 48 h of ICU stay vs. those not. First-line antifungal therapy was analyzed only for patients for whom the positivity of the blood culture was known before death and was categorized into four groups: “fluconazole”, “echinocandins” (aggregating all three drugs available), “other treatments” (all other antifungal drugs and drug combinations), and “no treatment” (no antifungal therapy).
Statistical analysis
Incidence rates were calculated per 10,000 hospitalization days using annual hospital activity data available since 2004 (SAE administrative data, Ministry of Health, DREES). Univariate analysis was based on χ 2 or Fisher’s exact test when needed for discrete variables. χ 2 test for trends was used to determine trends over time in crude mortality and treatments rates, and incidences of candidemia. In order to identify risks factors for fungemia due to non-albicans Candida species, a multivariate multinomial regression analysis was performed using C. albicans as reference. The year of inclusion and all baseline variables representing preexisting conditions of patients before fungemia were introduced into the model. To identify risk factors associated with death in ICU during fungemia (crude death rate), a mixed logistic regression model was used, to account for dependency of measurement within hospitals and adjusted on the basis of the year of inclusion. Three models were built for the three outcomes evaluated, i.e., overall death (at day 30), early death (before day 8), and delayed death (between day 8 and day 30). All covariates associated with the outcome in univariate analysis with a p value less than 0.25 were introduced into the multivariate model to identify covariates significantly associated with the outcome (p < 0.05) using a backward stepwise procedure. Survivals were compared by the logrank test. Data were analyzed using Stata Statistical Software (version 12.0; College Station, TX).
Results
Characteristics of the population
During the study period, 2,507 patients had candidemia including 95 who developed 122 recurrences. A total of 2,571 isolates were recovered during the incident episodes related mainly to single species (2,424/2,571, 94.3 %). Candidemia occurred in ICU in 48.1 % of the cases and major characteristics of ICU hospitalized patients are provided in Table 1. Briefly, the majority were male (62 %), with 518/1,206 (43.0 %) patients aged at least 65 years. Surgery within the past 30 days was found in 48.1 % of the patients with digestive tract and cardiovascular surgery representing 45.2 % and 20.7 % of surgical procedures, respectively. C. albicans was involved in 57.1 % of the cases. In ICU, 19.8 % (96/484) of patients developed candidemia within 48 h following admission. Their characteristics did not differ from those developing candidemia later on in terms of age, sex, allogeneic HSCT, transplantation, hematological malignancy, arterial catheter, prior exposure to antifungals, species, and outcome.
When comparing ICU vs. non-ICU patients, the former had more frequent recent surgery and central venous and arterial catheters, were more often preexposed to fluconazole and treated with echinocandin, and were less frequently infected with C. parapsilosis.
Outcome in patients with single species candidemia
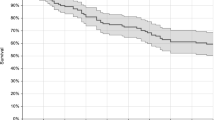
ICU patients had a higher overall death rate (51 %) than non-ICU patients (30.7 %) (p < 0.001) and this was also true when considering early deaths (before day 8) (Table 1). In ICU, the survival rate of patients infected with C. parapsilosis was higher (logrank test p = 0.0005) and that of patients infected with C. krusei (logrank test p = 0.0148) or C. kefyr (logrank test p = 0.0267) lower compared to patients infected by C. albicans (Fig. 1a). Outside ICU, the survival rate of patients infected with C. parapsilosis was also higher (logrank test p = 0.0018) and that of patients infected with C. krusei (logrank test p = 0.0267) also lower compared to patients infected by C. albicans (Fig. 1b). All first-line antifungal treatments (fluconazole, echinocandins, or others) were associated with a similar survival (data not shown). The odds of death in ICU was then analyzed according to its timing in patients with single infections using logistic regression analysis (Table 2). Independent risk factors for death at 30 days were age, presence of arterial catheter, infection by C. kefyr, preexposure to caspofungin, and lack of antifungal therapy given in those for whom the positivity of the blood culture was known before death and/or for whom the information about treatment was recorded (p < 0.05). In contrast, recent surgery and infection caused by C. parapsilosis or C. glabrata were protective. The factor associated with the highest odds of death (11.04) was lack of initial antifungal treatment when considering early death before day 8.
Factors associated with infection by non-albicans Candida species or by multiple species
Since infections due to some non-albicans species are associated with specific management recommendations, we analyzed by multinomial regression analysis the odds of being infected with a given species in comparison with that of being infected with C. albicans in ICU hospitalized patients (Table 3). The odds of infection by C. glabrata were increased in older patients and in case of recent exposure to fluconazole or caspofungin. The odds of infection by C. parapsilosis were increased in patients with recent caspofungin exposure. The odds of being infected by C. krusei were reduced in case of recent surgery, and increased in patients with solid tumor or solid organ transplantation, and recent exposure to fluconazole or caspofungin. The odds associated with C. tropicalis candidemia were the presence of hematological disorders and recent exposure to fluconazole while the odds were reduced in case of solid tumor. C. kefyr was associated with recent exposure to caspofungin. Finally, odds associated with candidemia due to multiple species were caspofungin preexposure and active intravenous drug addiction.
Trends over the last decade
The incidence of candidemia in the overall population and in ICU patients increased overtime (p = 0.01 and p = 0.0001, respectively) (Fig. 2). In the overall population, C. albicans and C. glabrata were responsible for this increase (from 0.55 in 2004 to 0.64 episodes/10,000 days of hospitalization in 2009 (p = 0.02) and from 0.16 in 2004 to 0.22 episodes/10,000 days of hospitalization in 2009 (p = 0.01), respectively) while the incidence of candidemia due to other species remained unchanged during the study period. In ICU patients only C. albicans incidence increased from 4.72 in 2004 to 6.31 episodes/10,000 days of hospitalization in 2009 (p = 0.005).
The major characteristics of the patients, including the proportion of older patients, did not change between 2002 and 2010 (data not shown). Preexposure to fluconazole did not change over time, while that of caspofungin increased in ICU patients (p = 0.0004, data not shown). In ICU, prescription of fluconazole decreased over time (from 64.8 to 38.8 % in the first and last year of study, respectively, p < 0.0001), while that of echinocandins increased over time (from 4.6 to 48.5 % in the first and last year of study, respectively, p < 0.0001).
Finally, the overall death rate increased over time in ICU from 42.7 % in 2003 to 53.9 % in 2009 (p < 0.003) but remained unchanged in the overall population (Fig. 2).
Discussion
On the basis of our analysis of a population whose characteristics are similar to those found in a recent multicenter study of septic shocks attributable to Candida spp. [16], a significant increasing incidence of candidemia was observed together with an increasing mortality rate in ICU. This was observed through a large prospective multicentric hospital-based surveillance program implemented over almost a decade in the Paris area. Even if we are fully aware that the epidemiology of candidemia is highly variable in Europe and elsewhere, these trends are worrisome in the context of expanded antifungal armamentarium, published management guidelines, and better diagnostic tools available at least in large tertiary care centers such as those involved in the present study [17, 18].
Of importance, similar incidence trends for candidemia and even other invasive fungal infections were observed recently using national registries in Europe [14, 19]. Indeed, the epidemiology of candidemia that we described here did not differ drastically from recently published data even if some differences can be noted. The global proportion of C. albicans was higher than in two recent North American studies (56 vs. 38–42.1 %) [20, 21]. That of C. glabrata was lower here (13.6 %) than in several recent studies reporting at least 20 % of C. glabrata among isolates causing fungemia [6, 20, 21]. C. parapsilosis ranked second in Spain, Brazil, and Italy and third here [13, 22, 23]. Of note, relative to ICU patients infected with C. albicans, those with C. parapsilosis were less likely to be hospitalized in ICU, those with C. tropicalis were more likely to have hematological disorders and less likely solid tumor, and those with C. krusei were more likely to have a solid tumor and solid organ transplantation but less recent surgery.
The increased incidence found here concerned two species (C. albicans and C. glabrata) at least in the overall population and only C. albicans in ICU. A specific increase in C. glabrata fungemia was recently reported in the USA, Europe, and Brazil [20, 24, 25]. We have no explanation for the increased incidence of C. albicans. We know that species involved can largely be influenced by ecosystems and local management of the patients at risk. In a recent study, 15 % of the patients were preexposed to antifungal, including fluconazole in 64 % and echinocandins in 15 % [7], while in another one, preexposure concerned up to 43.4 % of the patients [21] especially (more than 67 % of the cases) those with breakthrough episodes [12]. Here, preexposure concerned 10.6 % of patients hospitalized in ICU. Preexposure to fluconazole did not change over time, while that of caspofungin increased in ICU. We and others have previously evidenced the impact of prolonged prior fluconazole exposure on the distribution of Candida species [15, 24, 26]. Interestingly, in the current multinomial analysis performed in ICU patients with C. albicans as the reference, preexposure to fluconazole was an independent factor of infection with C. krusei, C. tropicalis, or C. glabrata, whereas preexposure to caspofungin was an independent factor of infection with C. parapsilosis, C. krusei, C. kefyr, C. glabrata, and mixed infections, thereby emphasizing the broader than expected ecological impact of echinocandin exposure [15, 27, 28]. It should also be remembered that in addition to the emergence of potentially more virulent Candida species induced by echinocandin exposure such as C. krusei and C. kefyr found here, the emergence of echinocandin resistance among usually susceptible Candida species is also worrisome [29, 30].
Our large database allowed us to focus on trends in death and look for explanations of the increased death rate over time in the overall population and in ICU. The first explanation could be the species involved. Here and in other studies [3, 13, 21, 31], C. parapsilosis and C. krusei/C. kefyr were associated with the lowest and highest death rates, respectively, while others found an increased death rate among patients with C. tropicalis or C. glabrata fungemia [32, 33]. However, the mortality trends cannot be explained by the evolving distribution of C. parapsilosis or C. krusei/C. kefyr. Another explanation might have been an increased proportion of elderly patients over time in ICU. However, it remained stable yet high (31.8 and 11.1 % of episodes occurred in patients aged at least 65 years and at least 80 years, respectively) as already noted by others with the highest incidence rates of candidemia in adults over 65 [7]. The death rate is also known to depend on underlying diseases or settings, as found here in ICU patients (51 %) and as already reported [14]. However, although mortality remained stable for the overall population, the most worrying finding is its increase in ICU. Several recent studies reported changes in the case-mix of critically ill patients. Nationwide trends of severe sepsis in the USA showed that from 2000 to 2007, more patients had at least three organ system failures [34]. In an Australian tertiary ICU, there was a significant increase in severity of illness and Charlson comorbidity index of the patients over a 16-year study period [35]. Finally, although we did not capture this information during our large prospective surveillance program, unpublished data from ICUs which participated in the present study indicate that the simplified acute physiology score II score and the percentage of mechanically ventilated patients increased from 37, 42 to 44, and from 41, 49, to 60 % in 2000, 2005, and 2010, respectively [CubRea Network (“Collège des Utilisateurs des Bases de Données en Réanimation”), Ile de France; Biostatistique et Informatique Médicale, Hôpital Ambroise Paré, Boulogne, France]. However, a recent study showed an average annual increase in the incidence of severe sepsis of 13 % between 2004 and 2009 while in-hospital death decreased from 35 to 25.6 % across the 6-year period [36]. In addition, a recent meta-analysis of 36 multicenter severe sepsis trials, with a total of 14,418 participants, found a decreasing mortality of 3.0 % annually (95 % CI, 0.8–5.0 %; p = 0.009), decreasing from 46.9 % during years 1991–1995 to 29 % during years 2006–2009 [37]. Thus, it can be speculated that more ICU patients now survive from bacterial sepsis and could be further exposed to life-threatening ICU-acquired candidemia.
The lack of overall diminution and a fortiori the increased death rate found in ICU patients are shocking knowing that new antifungals became available and that several guidelines for the management of candidemia were published during the same period. Among ICU patients, 55.4 % were treated with fluconazole and 26.2 % with an echinocandin, a percentage close to what was reported recently in the USA [7] even if more echinocandins were prescribed in one study [31]. Other studies found that receiving an antifungal was an independent factor decreasing both early and late death [22] and that echinocandins as first-line therapy reduced the death rate [38]. In the present study, outcome did not change according to initial antifungal therapy, and only lack of initial antifungal therapy impacted both overall and early deaths. Moreover, preexposure to caspofungin had a negative impact on the outcome. Although numerous other factors not captured here may have influenced the outcome, such as the delayed initiation of adequate antifungal therapy [8, 39, 40], although controversial [16], lack of consideration of antifungal dosing issues [40–42], and inadequate source control as recently emphasized [16], both the strong ecological impact and potentially its deleterious effect on outcome emphasize the need for not only triazole but also echinocandin stewardship in our hospitals.
In conclusion we observed an increase in the incidence of candidemia and its death rate in ICU. The causes of these worrisome trends merit further studies.
References
Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, Garbino J, Calandra T, Glauser MP, Tauber MG, Pittet D (2004) Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis 38:311–320
Marra AR, Camargo LF, Pignatari AC, Sukiennik T, Behar PR, Medeiros EA, Ribeiro J, Girao E, Correa L, Guerra C, Brites C, Pereira CA, Carneiro I, Reis M, de Souza MA, Tranchesi R, Barata CU, Edmond MB (2011) Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J Clin Microbiol 49:1866–1871
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317
Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C (2005) The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41:1232–1239
Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163
Arendrup MC, Sulim S, Holm A, Nielsen L, Nielsen SD, Knudsen JD, Drenck NE, Christensen JJ, Johansen HK (2011) Diagnostic issues, clinical characteristics, and outcomes for patients with fungemia. J Clin Microbiol 49:3300–3308
Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM (2012) Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis 55:1352–1361
Kollef M, Micek S, Hampton N, Doherty JA, Kumar A (2012) Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 54:1739–1746
Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ (2012) ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):19–37
Chow JK, Golan Y, Ruthazer R, Karchmer AW, Carmeli Y, Lichtenberg DA, Chawla V, Young JA, Hadley S (2008) Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med 36:1993–1998
Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP (2009) Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115:4745–4752
Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C (2011) Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS ONE 6:e24198
Arendrup MC, Dzajic E, Jensen RH, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Leitz C, Lemming LE, Nielsen L, Olesen B, Rosenvinge FS, Roder BL, Schonheyder HC (2013) Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect 19:E343–E353
Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F (2011) Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother 55:532–538
Bassetti M, Righi E, Ansaldi F, Merelli M, Cecilia T, De Pascale G, Diaz-Martin A, Luzzati R, Rosin C, Lagunes L, Trecarichi EM, Sanguinetti M, Posteraro B, Garnacho-Montero J, Sartor A, Rello J, Rocca GD, Antonelli M, Tumbarello M (2014) A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 40:839–845
Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL (2013) Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 57:1237–1245
Leon C, Ostrosky-Zeichner L, Schuster M (2014) What’s new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med 40:808–819
Asmundsdottir LR, Erlendsdottir H, Gottfredsson M (2013) Nationwide study of candidemia, antifungal use, and antifungal drug resistance in Iceland, 2000 to 2011. J Clin Microbiol 51:841–848
Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T (2012) Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two US cities from 2008 to 2011. J Clin Microbiol 50:3435–3442
Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D (2012) Epidemiology and outcomes of candidemia in 3648 patients: data from the prospective antifungal therapy (PATH Alliance®) registry, 2004–2008. Diagn Microbiol Infect Dis 74:323–331
Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez M, Saballs P, Fridkin SK, Morgan J, Rodriguez-Tudela JL, Warnock DW, Pahissa A (2005) Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 43:1829–1835
Colombo AL, Nucci M, Park BJ, Nouer SA, Arthington-Skaggs B, da Matta DA, Warnock D, Morgan J (2006) Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol 44:2816–2823
Chow JK, Golan Y, Ruthazer R, Karchmer AW, Carmeli Y, Lichtenberg D, Chawla V, Young J, Hadley S (2008) Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis 46:1206–1213
Moretti ML, Trabasso P, Lyra L, Fagnani R, Resende MR, de Oliveira Cardoso LG, Schreiber AZ (2013) Is the incidence of candidemia caused by Candida glabrata increasing in Brazil? Five-year surveillance of Candida bloodstream infection in a university reference hospital in southeast Brazil. Med Mycol 51:225–230
Rodriguez D, Almirante B, Cuenca-Estrella M, Rodriguez-Tudela JL, Mensa J, Ayats J, Sanchez F, Pahissa A (2010) Predictors of candidaemia caused by non-albicans Candida species: results of a population-based surveillance in Barcelona, Spain. Clin Microbiol Infect 16:1676–1682
Blanchard E, Lortholary O, Boukris-Sitbon K, Desnos-Ollivier M, Dromer F, Guillemot D (2011) Prior caspofungin exposure in patients with hematological malignancies is a risk factor for subsequent fungemia due to decreased susceptibility in Candida spp.: a case-control study in Paris, France. Antimicrob Agents Chemother 55:5358–5361
Fournier P, Schwebel C, Maubon D, Vesin A, Lebeau B, Foroni L, Hamidfar-Roy R, Cornet M, Timsit JF, Pelloux H (2011) Antifungal use influences Candida species distribution and susceptibility in the intensive care unit. J Antimicrob Chemother 66:2880–2886
Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, Grenouillet F, Cassaing S, Baixench MT, Bretagne S, Dromer F, Lortholary O (2012) Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg Infect Dis 18:86–90
Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA (2013) Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732
Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM (2009) Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48:1695–1703
Munoz P, Giannella M, Fanciulli C, Guinea J, Valerio M, Rojas L, Rodriguez-Creixems M, Bouza E (2011) Candida tropicalis fungaemia: incidence, risk factors and mortality in a general hospital. Clin Microbiol Infect 17:1538–1545
Slavin MA, Sorrell TC, Marriott D, Thursky KA, Nguyen Q, Ellis DH, Morrissey CO, Chen SC (2010) Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J Antimicrob Chemother 65:1042–1051
Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, Jacobs E, Nanchal R (2011) Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 140:1223–1231
Williams TA, Ho KM, Dobb GJ, Finn JC, Knuiman MW, Webb SA (2010) Changes in case-mix and outcomes of critically ill patients in an Australian tertiary intensive care unit. Anaesth Intensive Care 38:703–709
Gaieski DF, Edwards JM, Kallan MJ, Carr BG (2013) Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41:1167–1174
Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ (2014) Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med 42:625–631
Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ (2012) Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 54:1110–1122
Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT (2006) Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31
Zilberberg MD, Kollef MH, Arnold H, Labelle A, Micek ST, Kothari S, Shorr AF (2010) Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis 10:150
Labelle AJ, Micek ST, Roubinian N, Kollef MH (2008) Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 36:2967–2972
Pai MP, Turpin RS, Garey KW (2007) Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob Agents Chemother 51:35–39
Acknowledgments
The authors thank all the physicians and technicians in the 24 hospitals who made this study possible. The YEASTS program was supported in part by Institut de Veille Sanitaire and Institut Pasteur. The funders had no role in study design, data collection, analysis or interpretation of data.
Conflicts of interest
OL and MW: member of speaker’s bureau Merck, Pfizer, Astellas, Gilead. SB: member of speaker’s bureau Gilead. FD, CR, LD, KS, and AF: none
Ethical standards
The research described herein was carried out in compliance with French law and the Declaration of Helsinki (as adopted in 2000), and was approved by the Institut Pasteur institutional review board (IRB #2009-34). Approval of the “Commission Nationale de l’Informatique et des Libertés” was obtained, ensuring that the patients’ data were kept anonymous according to French regulations.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Additional information
The group’s members are listed in the Appendix.
Take-home message: Neither the availability of new antifungals nor the publication of numerous guidelines prevented an increase of C. albicans candidemia and death in ICU patients between 2002 and 2010 in the Paris area.
Appendix
Appendix
The following investigators participated in the YEASTS program of the French Mycosis Study Group: collection of data in each participating center: C. Bouges-Michel (hôpital Avicenne, Bobigny), I. Poilane (hôpital Jean Verdier, Bondy), J. Dunan (hôpital Ambroise Paré, Boulogne), G. Galeazzi (hôpital Louis Mourier, Colombes), A. Alanio, F. Foulet (hôpital Henri Mondor, Créteil), N. Fauchet (Centre Intercommunal, Créteil), E. Forget (hôpital Beaujon, Clichy), C. Lawrence (hôpital Raymond Poincaré, Garches), A. Angoulvant, C. Bonnal, C. Hennequin, F. Botterel (hôpital du Kremlin Bicêtre, Kremlin-Bicêtre), O. Eloy (Centre Hospitalier, Le Chesnay), M.-F. David, N. Khassis, L. Milhaila (hôpital Paul Brousse, Villejuif), E. Chachaty (Institut Gustave Roussy, Villejuif), and in Paris: C. Chochillon (hôpital Bichat), F. Lesle, A. Paugam, M.-T. Baixench (hôpital Cochin), M.-C. Escande (Institut Curie), M. Cornet (Hôtel Dieu), M.-E. Bougnoux, Y. Sterckers, S. Challier (hôpital Necker), E. Dannaoui, V. Lavarde (hôpital Européen Georges Pompidou), A. Datry, B. L. Mimouni, S. Brun, A. Fekkar (hôpital de la Pitié-Salpétrière), J. Guitard, J.-L. Poirot (hôpital Saint Antoine), C. Lacroix (hôpital Saint Louis, Fernand Widal et Lariboisière), D. Moissenet (hôpital Trousseau), M. Develoux (hôpital Tenon), P. Mariani, S. Bonacorsi (hôpital Robert Debré). Technical analysis of the isolates at the National Reference Center for Invasive Mycoses and Antifungals: Marie Desnos-Ollivier, Dea Garcia-Hermoso, Damien Hoinard, Dorothée Raoux.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lortholary, O., Renaudat, C., Sitbon, K. et al. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med 40, 1303–1312 (2014). https://doi.org/10.1007/s00134-014-3408-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3408-3