Abstract
Objective
Lipoproteins modulate vascular cell function in inflammation. In this study, we analyzed whether plasma concentrations of lipoproteins and apolipoproteins in human sepsis are related to patient survival and the activation of blood monocytes and platelets.
Design
Observational study.
Setting
Medical and surgical intensive care units (ICU) of a university hospital.
Patients
151 consecutive patients after sepsis criteria had been met for the first time.
Interventions
None.
Measurements
Plasma lipoproteins, apolipoproteins, platelet CD62P-expression, monocyte HLA-DR-expression, SAPS II-scores (Simplified Acute Physiology Score) and 30-day-mortality were recorded.
Results
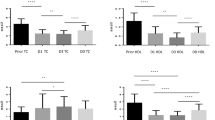
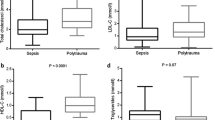
Total cholesterol, high-density-lipoprotein (HDL) and low-density-lipoprotein (LDL) cholesterol, apolipoprotein (apo)-AI and apo-B were all found to be significantly lower in non-survivors than in survivors. In contrast to other (apo)lipoproteins, apo-AI and HDL cholesterol further decreased in non-survivors during the ICU stay. Logistic regression analysis revealed apo-AI to be an independent predictor of 30-day-mortality. A significant inverse correlation was found for apo-AI/HDL-cholesterol and platelet activation. Later in the course of the disease, HLA-DR expression on monocytes correlated positively to apo-AI and apo-CI concentrations and inversely to the apo-E concentration.
Conclusion
Low apo-AI is independently related to 30-day mortality in human sepsis and the decrease in apo-AI/HDL cholesterol correlates to increased platelet activation. Moreover, changes in apolipoproteins supposed to modulate lipopolysaccharide effects, such as apo-CI and apo-E, correlate to monocyte activation.

Similar content being viewed by others
References
Hoesel LM, Gao H, Ward PA (2006) New insights into cellular mechanisms during sepsis. Immunol Res 34:133–141
Murch O, Collin M, Hinds CJ, Thiemermann C (2007) Lipoproteins in inflammation and sepsis. I. Basic science. Intensive Care Med 33:13–24
Ma J, Dempsey AA, Stamatiou D, Marshall KW, Liew CC (2007) Identifying leukocyte gene expression patterns associated with plasma lipid levels in human subjects. Atherosclerosis 191:63–72
Berbee JF, Havekes LM, Rensen PC (2005) Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J Endotoxin Res 11:97–103
Birjmohun RS, van Leuven SI, Levels JH, van ‘t Veer C, Kuivenhoven JA, Meijers JC, Levi M, Kastelein JJ, van der Poll T, Stroes ES (2007) High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler Thromb Vasc Biol 27:1153–1158
McDonald MC, Dhadly P, Cockerill GW, Cuzzocrea S, Mota-Filipe H, Hinds CJ, Miller NE, Thiemermann C (2003) Reconstituted high-density lipoprotein attenuates organ injury and adhesion molecule expression in a rodent model of endotoxic shock. Shock 20:551–557
Mineo C, Shaul PW (2003) HDL stimulation of endothelial nitric oxide synthase: a novel mechanism of HDL action. Trends Cardiovasc Med 13:226–231
Nofer JR, Walter M, Kehrel B, Seedorf U, Assmann G (1998) HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1, 2-diacylglycerol and inositol 1, 4, 5-tris-phosphate. Arterioscler Thromb Vasc Biol 18:861–869
Lerch PG, Spycher MO, Doran JE (1998) Reconstituted high density lipoprotein (rHDL) modulates platelet activity in vitro and ex vivo. Thromb Haemost 80:316–320
Liao XL, Lou B, Ma J, Wu MP (2005) Neutrophils activation can be diminished by apolipoprotein A-I. Life Sci 77:325–335
Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J (2008) High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 28:2071–2077
Van Oosten M, Rensen PC, Van Amersfoort ES, Van Eck M, Van Dam AM, Breve JJ, Vogel T, Panet A, Van Berkel TJ, Kuiper J (2001) Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J Biol Chem 276:8820–8824
Rensen PC, Oosten M, Bilt E, Eck M, Kuiper J, Berkel TJ (1997) Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats In vivo. J Clin Invest 99:2438–2445
de Bont N, Netea MG, Demacker PN, Kullberg BJ, van der Meer JW, Stalenhoef AF (2000) Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur J Clin Invest 30:818–822
Li Y, Dong JB, Wu MP (2008) Human ApoA-I overexpression diminishes LPS-induced systemic inflammation and multiple organ damage in mice. Eur J Pharmacol 590:417–422
Berbee JF, van der Hoogt CC, Kleemann R, Schippers EF, Kitchens RL, van Dissel JT, Bakker-Woudenberg IA, Havekes LM, Rensen PC (2006) Apolipoprotein CI stimulates the response to lipopolysaccharide and reduces mortality in gram-negative sepsis. FASEB J 20:2162–2164
Schippers EF, Berbée JF, van Disseldorp IM, Versteegh MI, Havekes LM, Rensen PC, van Dissel JT (2008) Preoperative apolipoprotein CI levels correlate positively with the proinflammatory response in patients experiencing endotoxemia following elective cardiac surgery. Intensive Care Med 34:1492–1497
Berbée JF, Mooijaart SP, de Craen AJ, Havekes LM, van Heemst D, Rensen PC, Westendorp RG (2008) Plasma apolipoprotein CI protects against mortality from infection in old age. J Gerontol A Biol Sci Med Sci 63:122–126
Chien JY, Jerng JS, Yu CJ, Yang PC (2005) Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med 33:1688–1693
Vermont CL, den Brinker M, Kakeci N, de Kleijn ED, de Rijke YB, Joosten KF, de Groot R, Hazelzet JA (2005) Serum lipids and disease severity in children with severe meningococcal sepsis. Crit Care Med 33:1610–1615
Barlage S, Frohlich D, Bottcher A, Jauhiainen M, Müller HP, Noetzel F, Rothe G, Schütt C, Linke RP, Lackner KJ, Ehnholm C, Schmitz G (2001) ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. J Lipid Res 42:281–290
Chenaud C, Merlani PG, Bandshapp O, Ricou B (2006) Serum lipids or apolipoprotein A-I and disease severity of meningococcal sepsis. Crit Care Med 34:270–271
American College of Chest Physicians/Society of Critical Care Medicine (1992) Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Reinhart K, Brunkhorst F, Bone H, Gerlach H, Gründling M, Kreymann G, Kujath P, Marggraf G, Mayer K, Meier-Hellmann A, Peckelsen C, Putensen C, Quintel M, Ragaller M, Rossaint R, Stüber F, Weiler N, Welte T, Werdan K, Deutsche Sepsis-Gesellschaft eV (2006) Diagnosis and therapy of sepsis: guidelines of the German Sepsis Society Inc. and the German Interdisciplinary Society for Intensive and Emergency Medicine. Anaesthesist 55(Suppl 1):43–56
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Walldius G, Jungner I (2007) Apolipoprotein A-I versus HDL cholesterol in the prediction of risk for myocardial infarction and stroke. Curr Opin Cardiol 22:359–367
Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Müller JM, Döcke WD, Kox WJ (1996) Monocyte deactivation-rationale for a new therapeutic strategy in sepsis. Intensive Care Med 22(Suppl 4):S474–S481
Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, Lösche W (2002) Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock 17:263–268
Florentin M, Liberopoulos EN, Wierzbicki AS, Mikhailidis DP (2008) Multiple actions of high-density lipoprotein. Curr Opin Cardiol 23:370–378
Barlage S, Boettcher D, Boettcher A, Dada A, Schmitz G (2006) High density lipoprotein modulates platelet function. Cytometry A 69:196–199
Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G (2006) Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med 32:75–79
Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, Amichay D, Almog Y (2009) The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med 35:1255–1260
Pajkrt D, Doran JE, Koster F (1996) Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med 184:1601–1608
Galbois A, Thabut D, Tazi KA, Rudler M, Mohammadi MS, Bonnefont-Rousselot D, Bennani H, Bezeaud A, Tellier Z, Guichard C, Coant N, Ogier-Denis E, Moreau R, Lebrec D (2009) Ex vivo effects of high-density lipoprotein exposure on the lipopolysaccharide-induced inflammatory response in patients with severe cirrhosis. Hepatology 49:175–184
Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR (2005) Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ Res 97:236–243
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barlage, S., Gnewuch, C., Liebisch, G. et al. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med 35, 1877–1885 (2009). https://doi.org/10.1007/s00134-009-1609-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1609-y