Abstract
Objective
The aim of this study was to evaluate the correlation between sympathetic nervous activation and the immune response in patients following subarachnoid haemorrhage (SAH).
Design and setting
Clinical study in a neurosurgical intensive care unit.
Patients and participants
Fourteen patients with acute non-traumatic SAH were included. Fifteen healthy, age-matched volunteers served as controls for measurement of catecholamine spillover.
Intervention
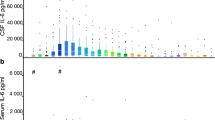
Blood sampling for C3a, C5b-9, IL-6, IL-8 and norepinephrine kinetic determination was made within 48 h, at 72 h and on the 7th–10th day after the SAH.
Measurements and results
SAH patients exhibited a profound increase in the rate of norepinephrine spillover to plasma at 48 h, 72 h and 7–10 days after the insult, 3–4 times that in healthy individuals. The plasma levels of C3a, IL-6 and C5b-9 were significantly elevated at 48 h, at 72 h and 7–10 days after the SAH, but the plasma level of IL-6 decreased significantly 7–10 days after the SAH. There was no relationship between the magnitude of sympathetic activation and the levels of inflammatory markers.
Conclusions
Following SAH a pronounced activation of the sympathetic nervous system and the inflammatory system occurs. The lack of significant association between the rate of spillover of norepinephrine to plasma and the plasma levels of inflammatory markers indicates that the two processes, sympathetic activation and the immune response, following SAH are not quantitatively linked. In spite of a persistent high level of sympathetic activation the plasma level of IL-6 decreased significantly one week after SAH.

Similar content being viewed by others
References
Moynihan J, Kruszewska B, Madden K, Callahan T (2004) Sympathetic nervous system regulation of immunity. J Neuroimmunol 147:87–90
Elenkov I, Wilder R, Chrousos G, Vizi S (2000) The sympathetic nerve – an integrative interface between two super systems: the brain and the immune system. Pharmacol Rev 52:595–638
Maier S, Watkins L (1998) Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behaviour, mood and cognition. Psychol Rev 105:83–107
Watkins L, Maier S (2005) Immune regulation of central nervous system functions. From sickness responses to pathological pain. J Intern Med 257:139–155
Felten D, Felten S, Bellinger D, Carlsson S, Ackerman K, Madden K, Olschowki J, Livnat S (1987) Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev 100:225–260
Felten D, Felten S, Bellinger D, Madden K (1993) Fundamental aspects of neural-immune signaling. Psychother Psychosom 60:46–56
Black P (2002) Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun 16:622–653
Connor T, Brewer C, Kelley J, Harkin A (2005) Acute stress response suppresses pro-inflammatory cytokines TNF-alfa and IL-1beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol 159:119–128
Volk T, Döpfmer U, Schmutzler M, Rimpau S, Schnitzler H, Konertz W, Hoeflich C, Döcke W, Spies C, Volk H, Kox W (2003) Stress induced IL-10 does not seem to be essential for early monocyte deactivation following cardiac surgery. Cytokine 24:237–243
van der Poll T, Jansen J, Endert E, Sauerwein H, van Deventer S (1994) Noradrenaline inhibits lipopolysaccharide-induced tumour necrosis factor and interleukin 6 production in human whole blood. Infect Immun 62:2046–2050
Bürger A, Benicke M, Deten A, Zimmer HG (2001) Catecholamines stimulate interleukin-6 synthesis in rat cardiac fibroblasts. AJP Heart 281:14–21
Meisel C, Schwab JM, Prass K, Meisel A, Dirnag U (2005) Central nervous system injury induced immune deficiency syndrome. Nat Rev/Neuroscience 6:775–786
Dhabhar F (2003) Stress, leukocyte trafficking, and the augmentation of skin immune function. Ann N Y Acad Sci 992:205–217
Dhabhar F, McEven B (1997) Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leucocyte trafficking. Brain Behav Immun 11:286–306
Tracey K (2002) The inflammatory reflex. Nature 420:853–859
Macmillan C, Grant I, Andrews P (2002) Pulmonary and cardiac sequelae of subarachnoid haemorrhage: time for active management? Intensive Care Med 28:1012–1023
Cruickshank JM, Neil-Dwyer G, Stott AW (1974) Possible role of catecholamines, corticosteroids and potassium in production of electrocardiographic abnormalities associated with subarachnoid haemorrhage. Br Heart J 36:697–706
Dilraj A, Botha JB, Rambiritch V, Miller R, van Dellen JR (1992) Levels of catecholamines in plasma and cerebrospinal fluid in aneurysmal subarachnoid hemorrhage. Neurosurg 31:42–51
Naredi S, Lambert G, Edén E, Zäll S, Runnerstam M, Rydenhag B, Friberg P (2000) Increased sympathetic nervous activity in patients with non-traumatic subarachnoid hemorrhage. Stroke 31:901–906
Dampney R, Moon E (1980) Role of ventrolateral medulla in vasomotor response to cerebral ischemia. Am J Physiol 239:349–358
Reis D, Morrison S, Ruggiero D (1988) The C1 area of the brainstem in tonic and reflex control of blood pressure. Hypertension 11:18–23
Sun M, Reis D (1994) Hypoxia selectively excites vasomotor neurons of rostral ventrolateral medulla in rats. Am J Physiol 266:R245–R256
Yoshimoto Y, Tanaka Y, Hoya K (2001) Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke 32:1989–1993
Hidetoshi K, Takashi S (1989) Activated complement components C3a and C4a in cerebrospinal fluid and plasma following subarachnoid hemorrhage. J Neurosurg 71:741–746
Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schutt S, Fritzinger M, Horn P, Vajkoczy P, Kreisel S, Brunner J, Schmiedek P, Hennerici M (2001) Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries Neurol Neurosurg Psychiatry 70:534–537
Mathiesen T, Andersen B, Loftenius A, von Holst H (1993) Increased interleukin-6 levels in cerebrospinal fluid following subarachnoid hemorrhage. J Neurosurg 78:562–567
Kasuya H, Shimizu T (1989) Activated complement components C3a and C4a in cerebrospinal fluid and plasma following subarachnoid hemorrhage. J Neurosurg 71:741–746
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6:1–9
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14–20
Eisenhofer G (2005) Sympathetic nerve function. Assessment by radioisotope dilution analysis. Clin Auton Res 15:264–283
Esler M, Jackmann G, Bobik A, Kelleher D, Jennings G, Leonard P, Skews H, Korner P (1979) Determination of norepinephrine apparent release and clearance in humans. Life Sci 25:1461–1470
Esler M, Jennings G, Leonard P Sacharias N, Burke F, Johns J, Blombery P (1984) Contribution of individual organs to total noradrenaline release in humans. Acta Physiol Scand 527:11–16
Padgett D, Glaser R (2003) How stress influences the immune response. Trends Immunol 24:444–448
Raßler B, Reißig C, Briest W, Tannapfel A, Zimmer HG (2003) Pulmonary edema and pleural effusion in norepinephrine-stimulated rats – hemodynamic or inflammatory effect? Mol Cell Biochem 250:55–63
Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M (1994) Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci 122:135–139
Van Beek J, Elward K, Gasque P (2003) Activation of complement in the central nervous system. Ann N Y Acad Sci 992:56–71
Acknowledgements
This study was performed at Sahlgrenska University Hospital, Göteborg, Sweden. The study was supported by grants from the Regional Health Care Authority of West Sweden, The Göteborg Medical Society, the Swedish Society of Medicine, the Swedish Medical Research Council and the National Health and Medical Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naredi, S., Lambert, G., Friberg, P. et al. Sympathetic activation and inflammatory response in patients with subarachnoid haemorrhage. Intensive Care Med 32, 1955–1961 (2006). https://doi.org/10.1007/s00134-006-0408-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0408-y