Abstract
Objective
Fibroproliferation markers like procollagen I predict mortality in patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). We sought to determine whether bronchoalveolar lavage fluid (BALF) from patients with lung injury contained mediators that would activate procollagen I promoter and if this activation predicted important clinical outcomes.
Design
Prospective controlled study of ALI/ARDS.
Setting
Intensive care units and laboratory of a university hospital.
Patients and participants
Acute lung injury/ARDS, cardiogenic edema (negative controls) and pulmonary fibrosis (positive controls) patients.
Interventions
Bronchoalveolar lavage fluid was collected within 48 h of intubation from ALI/ARDS patients. BALF was also collected from patients with pulmonary fibrosis and cardiogenic pulmonary edema. Human lung fibroblasts were transfected with a procollagen I promoter-luciferase construct and incubated with BALF; procollagen I promoter activity was then measured. BALF active TGF-β1 levels were measured by ELISA.
Results
Twenty-nine ARDS patients, nine negative and six positive controls were enrolled. BALF from ARDS patients induced 41% greater procollagen I promoter activation than that from negative controls (p<0.05) and a TGF-β1 blocking antibody significantly reduced this activation in ARDS patients. There was a trend toward higher TGF-β1 levels in the ARDS group compared to negative controls (−1.056 log10±0.1415 vs −1.505 log10±0.1425) (p<0.09). Procollagen I promoter activation was not associated with mortality; however, lower TGF-β1 levels were associated with more ventilator-free and ICU-free days.
Conclusions
Bronchoalveolar lavage fluid from ALI/ARDS patients activates procollagen I promoter, which is due partly to TGF-β1. Activated TGF-β1 may impact ARDS outcome independent of its effect on procollagen I activation.
Similar content being viewed by others
Introduction
The incidence of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) in the United States has been estimated to be 30 per 100,000, or nearly 150,000 cases annually [1]. Despite advances in supportive treatment and mechanical ventilation strategies, mortality in cases of ALI/ARDS remains approximately 40% [2] and patients who survive often have significant residual disability [3, 4]. At the time of death most patients are on mechanical ventilation, which may have contributed to the development of sepsis and/or multiple organ dysfunction syndrome [5], the most common cause of ARDS mortality [6].
Better to understand the pathophysiology of ARDS and to identify patients who might benefit from innovative therapies, investigators have sought measurements made early in the clinical course of patients with ARDS that predict mortality. Surprisingly, severity of hypoxemia, lung compliance abnormalities and markers of inflammation in the lung or blood all fail to predict mortality [7–9]. Several groups of investigators have demonstrated, however, that elevated markers of lung fibroproliferation predict a poor outcome in patients with ARDS [10–13]. For example, both the presence of fibrosis on lung biopsies [14, 15] and elevated levels of procollagen I and procollagen III in bronchoalveolar lavage fluid have been associated with increased risk of death in patients with ARDS [10–13]. In addition, the pulmonary dead space fraction, which may be a marker of fibrosis, was shown to predict mortality [16].
The factor(s) in ARDS responsible for the development of fibroproliferation are not known. We developed a bioassay that measures the activity of the human procollagen I promoter in primary cultures of normal human lung fibroblasts. We used this assay to determine whether bronchoalveolar lavage fluid (BALF) obtained from patients with ARDS within 48 h of intubation could activate procollagen I by transcription. We then measured active transforming growth factor-beta1 (TGF-β1), a member of a family of polypeptide growth factors that activates fibroblasts [17], in BALF and also determined whether blocking TGF-β1 could prevent BALF-induced procollagen I promoter activation. Lastly, we wished to determine if either of our biological assays could predict mortality or other important clinical outcomes. This work was presented at the American Thoracic Society International Conference, 2004 [18].
Methods
Study population
Subjects were recruited from the medical intensive care unit at Northwestern Memorial Hospital, a tertiary care hospital, between May 2001 and March 2003. Patients intubated within 48 h for acute respiratory failure, with bilateral infiltrates on chest X-ray, absence of clinical evidence of left atrial hypertension or (when available) a pulmonary artery wedge pressure below 18 mmHg and a PaO2/FIO2 ratio less than 300 were eligible for the study. Subjects intubated for cardiogenic pulmonary edema, and those undergoing elective bronchoscopy and bronchoalveolar lavage (BAL) without evidence of fibrotic lung disease were included as negative controls. A second group of patients with pulmonary fibrosis or stage IV sarcoidosis who were undergoing bronchoscopy with BAL were included as positive controls. ARDS subjects were followed until hospital discharge or death. Outcome measures included ventilator-free days, ICU-free days and survival. Informed consent was obtained from subjects or surrogates. The protocol was approved by the Institutional Review Board (IRB) of Northwestern University.
Bronchoalveolar lavage protocol
Each mechanically ventilated patient had BALF collected within 48 h of intubation. A fiberoptic bronchoscope or a BAL catheter was wedged into position in a distal bronchus and sterile saline was instilled in 20 cc aliquots and then aspirated and collected. This was repeated up to three times. The fluid was centrifuged at 1500 rpm within 30 min of collection for 10 min, aliquotted and frozen at −80°C.
Procollagen I promoter (PIP) reporter assay
Primary normal human lung fibroblasts (NHLF, Cambrex) were cultured in fibroblast growth medium 2 (FGM-2). Transient transfections of NHLF were carried out at 50% confluence in 6-well plates using TransIT-LT1 (Mirus) according to the manufacturer’s protocol. A typical transfection was performed by using 2.0 µg of a firefly luciferase reporter driven by procollagen I promoter (PIP) [19] and 50 ng of Renilla luciferase driven by herpes simplex virus thymidine kinase promoter (prL-TK vector, Promega). Cells were exposed to 1 ml of FGM-2 media along with either 1 ml of saline (negative control) or 1 ml of BALF from different samples. A positive control was conducted in cells exposed to 1 ml of FGF2-media combined with 1 ml of saline containing recombinant active human TGF-β1 (0.5 ng/ml). Cells were lysed using passive lysis buffer (Promega) and luciferase values were obtained using the Dual Luciferase Assay System (Promega). The ratio of PIP-luciferase/TK-luciferase was calculated for each sample and normalized to maximal activation as assessed by incubation with 0.5 ng/ml TGF-β1. Each BAL sample was analyzed three separate times and the average value was used for analysis. To determine whether inhibitors of PIP activation were present in the BALF, PIP activity was measured 24 h after the addition of exogenous active human TGF-β1 (0.5 ng/ml) to each BAL sample. In an available subset of five ARDS samples with the highest PIP activation, a neutralizing antibody for TGF-β1 (10 ng/ml, R&D systems) was added to the samples to determine whether it decreased PIP activation.
Bronchoalveolar lavage fluid procollagen I peptide assay
Procollagen I peptide was measured in BALF in a convenience sample of 11 ARDS patients and all negative controls by ELISA according to the manufacturer protocol (Metra CCIP, Quiedel).
Bronchoalveolar lavage fluid activated transforming growth factor-β1 assay
Active TGF-β1 was measured from BALF in duplicate using the TGFβ1 Emax ImmunoAssay System according to manufacturer protocol (Promega). This assay only measures TGF-β1 that has been cleaved and is biologically active.
Statistics
Demographic and physiologic variables are expressed as means ± SD. Continuous variables were analyzed using a Student’s t-test (SPSS for Windows 11.5; SPSS, Chicago, IL). Since active TGF-β1 values were not normally distributed, they were log transformed for analysis. One-way ANOVA was performed to determine if there was a significant difference between procollagen I promoter activation or log transformed active TGF-β1 mean values among three groups, and means between two groups were compared and p values calculated using Bonferroni’s correction. A paired t-test was performed to compare PIP activation for each dose of TGF-β1 as well as in the presence and absence of TGF-β1 antibody. Pearson correlation and Spearman correlation coefficients were calculated for PIP, TGF-β1 and outcome measures that were normally and not normally distributed, respectively. The highest third of TGF-β1 values were compared to the lowest two thirds for Kaplan-Meier analysis and calculation of odds ratios. A p value less than 0.05 was considered significant. For odds ratios, confidence intervals that did not cross were 1.0 were considered significant.
Results
Characteristics of the study population
Twenty-nine ARDS patients, nine negative control and six positive control patients were enrolled in the study. There were 20 (70.0%) men and 9 women in the ARDS group and the average age was 55±16 years. Other demographic variables for the ARDS patients are shown in Table 1 and clinical characteristics of negative and positive controls are shown in Table 2. The overall mortality was 41% (12/29). Non-survivors were significantly older and had higher MODS scores than survivors, but there were no differences in PaO2/FIO2 ratios, compliance or APACHE II scores (Table 3).
Bronchoalveolar lavage fluid from patients with acute respiratory distress syndrome induces procollagen I promoter activation
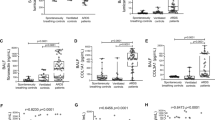
Mean PIP activation was significantly different among the three groups (Fig. 1; p<0.001). Further, the mean PIP values were significantly higher in positive compared to the negative controls, indicating that the assay was able to discriminate between patients with fibrotic and non-fibrotic lung disease. The mean PIP value was also significantly higher in ARDS patients than the mean in negative controls (p<0.05). In addition, the ARDS patients demonstrated greater variability in their PIP response, as evidenced by a significantly greater variance compared to negative or positive controls. There was nearly an 8-fold difference between ARDS subjects with the highest and lowest PIP activity (Fig. 11), and 4 of 29 (14%) had values within the fibrotic controls range or higher. Lastly, in a sub-sample of 11 ARDS patients for whom BAL fluid was available, procollagen I peptide levels were higher compared to those of negative controls (p<0.03) (Fig. 2). This sub-sample was not statistically different compared to the entire cohort of 29 ARDS patients with respect to age, APACHE II scores, PaO2/FIO2 ratios, compliance or outcome (data not shown).
Procollagen I promoter activation. Primary cultures of normal human lung fibroblasts were transiently transfected with plasmids encoding the human procollagen I promoter driving luciferase, which were then incubated with BALF from patients with ARDS, (-) controls, or (+) controls. Luciferase activity was measured in cell lysates 24 h later and is expressed as the percentage of the maximal (0.5 ng/ml TGF-β1) stimulated value. The horizontal bars represents sample means. p<0.001 for comparison between positive and negative controls and p<0.05 for comparison between ARDS and negative controls
Procollagen I peptide levels in ARDS patients (n=11) compared to negative controls. An ELISA assay was used to measure procollagen I peptide in the BALF from patients with ARDS and negative controls. The horizontal bars represents sample means. p<0.03 for comparison between ARDS patients and controls
Acute respiratory distress syndrome patients exhibit active transforming growth factor-β1
Mean TGF-β1 levels in the BALF of ARDS subjects were higher than the mean of negative control subjects (Fig. 3), though this did not reach statistical significance (p=0.09). As with PIP, there was significant variability in the active TGF-β1 levels of ARDS subjects with over a 3-log difference between the lowest and highest values. There was a significant correlation between TGF-β1 and procollagen I peptide values (p<0.05) (Fig. 4).
There is a trend toward higher active TGF-β1 levels in patients with ARDS than in controls. An ELISA assay was used to measure active TGF-β1 in the BALF from patients with ARDS or negative controls. All experiments were repeated in duplicate. The horizontal bars represents sample means. p<0.09 for comparison between ARDS patients and controls
Active transforming growth factor-β1 contributes to procollagen I promoter activation
The PIP-luciferase construct responded to TGF-β1 in a dose-dependent fashion (see electronic supplementary material) plateauing at a concentration of 0.5 ng/ml. In a group of five available patient BALs with the highest PIP activity, the addition of TGF-β1 blocking antibody significantly attenuated the PIP response (Fig. 5). The addition of exogenous TGF-β1 (0.5 ng/ml) to all BAL samples led to maximal PIP activation (see electronic supplementary material), suggesting the absence of significant TGF-β1 inhibitors in BALF.
TGF-β1 in the BALF is largely responsible for procollagen I promoter activation. Primary cultures of normal human lung fibroblasts were transiently transfected with the human procollagen I promoter- luciferase plasmid construct. They were then exposed to BALF from ARDS patients with the highest PIP activity (n=5) in the presence or absence of an antibody to TGF-β1 and luciferase activity was assessed 24 h later. There was a significant fall in procollagen I promoter activation in the presence of TGF-β1 antibody, p=0.008
Clinical outcomes
There was no significant correlation between of PaO2/FIO2 ratios, respiratory system compliance or APACHE II scores to outcome (Table 4) for either biological assay. In addition, there were no statistically significant differences in PIP values between ARDS survivors and non-survivors (Fig. 6A) and there was no significant correlation between PIP values and ICU-free (IFD) or ventilator-free days (VFD) (Table 4). In contrast, there was a significant negative correlation between active TGF-β1 levels and ventilator-free days and ICU-free days (Table 3). These results did not change when controlled for age and APACHE II scores. Furthermore, the odds ratios for prolonged mechanical ventilation (<14 VFD) and ICU stay (<14 IFD) with high TGF-β1 levels were 17.33 (2.35–127.34) and 6.50 (1.09–38.6), respectively. Mean TGF-β1 levels were higher in non-survivors compared to survivors (Fig. 6B), though this did not reach statistical significance (p=0.14). Survival curves stratified by TGF-β1 levels were not statistically different (Fig. 7).
Procollagen I promoter activation and active TGF-β1 levels and mortality. BALF procollagen I promoter activation was not different in patients who died from ARDS (28-day all cause mortality) and those who survived, p=0.56 (a). A similar analysis was conducted comparing Log active TGF-β1 levels in patients with ARDS (b). Non-survivors had higher mean levels than survivors, though the difference was not statistically significant, p=0.14
Discussion
In the present study we demonstrate that the BALF obtained from patients with ALI/ARDS within 48 h of intubation is capable of activating the human procollagen I promoter (PIP). This increase in procollagen I promoter activity appears to result from TGF-β1 present in the BALF, as active TGF-β1 was present in most ARDS subjects and PIP activation by BALF was blocked by TGF-β1 antibody. Several groups of investigators have detected procollagen I [12, 20, 21] and III [10, 11, 13, 22] in the BALF of patients with ARDS in the first 1–3 days following injury. Furthermore, BALF obtained from patients with ARDS 1–3 days following intubation is mitogenic for fibroblasts [13] and contains factors known to induce collagen production in some in vitro models including TGF-α [23] and TGF-β1 [24]. Many of these markers of fibroproliferation have been shown to predict poor outcomes in patients with ALI/ARDS [25].
We wished to determine whether the BALF from patients with ARDS was capable of activating the human procollagen I promoter and to determine which factor(s) were responsible for that activation. To address this question, we developed a bioassay using the full length human PIP in primary human lung fibroblasts. The use of primary cultured cells minimizes the effect of aberrant signaling mechanisms that may be present in immortalized cells. The use of the full length promoter allows for responses to both stimulatory and inhibitory cytokines that may be present in the BALF. Therefore, this system provides direct evidence for factor(s) in BALF from ARDS patients that can activate the PIP and also for the absence of significant inhibitors of this activation. Our bioassay differs from that used by Fahy et al., who transfected the human plasminogen activator inhibitor-1 (PAI-1) promoter [24] driving luciferase into mink lung epithelial cells. Their assay is very sensitive for detection of active TGF-β1, but cannot address the question of whether TGF-β1 or other cytokines is/are responsible for PIP activation.
An important role for TGF-β1 in the upregulation of fibroblasts following ALI would be consistent with previous human and experimental observations. TGF-β1 receptors can be detected in lung mesenchymal cells, microvascular endothelial cells as well as alveolar epithelial cells [26]. Total TGF-β1 expression is increased in experimental models of ALI and lung fibrosis [27, 28] and in patients with fibrotic lung diseases that appear similar to fibroproliferative ARDS histologically, such as idiopathic pulmonary fibrosis [29] and stage IV sarcoidosis [30]. Following the infant respiratory distress syndrome, patients who progressed to chronic lung disease had six-fold higher levels of active TGF-β1 than patients who did not [31].
Based on our analysis, TGF-β1 explains only about 50% of the procollagen I peptide levels in alveolar fluid. This suggests that factors other than TGF-β1 are present in alveolar fluid that also modulate procollagen I production. Potential candidates include thrombin [32], tissue plasminogen activator [33], insulin [34], insulin-like growth factor [35], IL-4 or IL-13 [36, 37].
The ability of the BALF collected from patients in the early phase of ARDS to activate the PIP activation was not associated with significant clinical outcomes; however, levels of active TGF-β1 are inversely correlated with ventilator-free days and ICU-free days. One previous study has shown that higher BAL procollagen I peptide levels were associated with increased mortality risk [12]. These investigators measured procollagen I peptide levels four times within the first week of intubation. While there were no differences in procollagen I levels between survivors and non-survivors in the first 24 h following intubation, there were significant differences by day 7. In contrast, day 1 BAL procollagen III levels have been shown to be predictive of mortality [10, 13] and day 1 tracheal aspirate levels of N-terminal procollagen III peptide levels greater than 1.75 U/ml were associated with a two-fold increased risk of death [11]. Collagen content and the ratio of type I to type III collagen is altered following lung injury [38] and it is possible that the balance of type I and type III collagen may be important in patient outcome. We did not measure BAL PIP activity later in ARDS (e.g. day 3 or day 7) and it may be that inability to downregulate procollagen 1 expression over time may impact outcome.
Our findings suggest that TGF-β1 levels may have a greater impact on outcome than PIP activation. BAL TGF-β1 levels inversely correlated with ventilator-free days and with ICU-free days. There is also a suggestion that patients with higher TGF-β1 levels may have a higher risk of death and may die faster, although neither outcome was statistically significant. The failure to reach statistical significance may be due to the relatively small numbers of patients in our study. Based on the unknown operating characteristics of our novel assays, it was difficult to calculate the power of our study a priori.
Our findings suggest that active TGF-β1 might act through mechanisms distinct from PIP activation and there is biologic plausibility in disassociating TGF-β1 effects on fibroproliferation and ARDS outcome. Several groups of investigators have reported that markers of alveolar epithelial cell injury predict outcome in patients with ARDS [39–41]. TGF-β1 can increase both alveolar epithelial permeability [42, 43] and downregulate alveolar epithelial proliferation [44]. TGF-β1 can also increase pulmonary endothelial permeability by promoting adherens junction disassembly [45] as well as inhibiting pulmonary endothelial proliferation [46]. Thus, the effect of TGF-β1 on alveolar epithelial and endothelial function in ARDS and its relationship to clinical outcome deserves further study.
One potential limitation of our study is the low volume of fluid used for our BALs, which may have limited our alveolar sampling. We used the same protocol, however, in our negative and positive controls and were able to detect differences in our patient groups. If anything, this under-sampling might have decreased our ability to detect factors that activate PIP or active TGF-β1. Thus, our results may underestimate TGF-β1 levels and the ability of the BALF to activate TGF-β1.
In conclusion, we demonstrate that active TGF-β1 in the BALF obtained from patients with ARDS within 48 h of intubation activates the procollagen I promoter in primary cultures of human lung fibroblasts. While the ability of the BALF to activate the procollagen I promoter does not predict clinical outcome, elevated levels of active TGF-β1 predict a poorer outcome.
References
Ware L, Matthay M (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
The ARDS Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. [see comments]. N Eng J Med 342:1301–1308
Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP (1999) Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 281:354–360
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–693
Slutsky AS, Tremblay LN (1998) Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 157:1721–1725
Montgomery AB, Stager MA, Carrico CJ, Hudson LD (1985) Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 132:485–489
Pittet JF, Mackersie RC, Martin TR, Matthay MA (1997) Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med 155:1187–1205
Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell CG, Bonde J (1999) Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark and Iceland. The ARF Study Group. Am J Respir Crit Care Med 159:1849–1861
Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA (1995) Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 152:1818–1824
Clark JG, Milberg JA, Steinberg KP, Hudson LD (1995) Type III procollagen peptide in the adult respiratory distress syndrome. Association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med 122:17–23
Chesnutt AN, Matthay MA, Tibayan FA, Clark JG (1997) Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med 156:840–845
Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A (1998) Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med 158:1432–1441
Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ (2000) Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med 162:1783–1788
Hill JD, Ratliff JL, Parrott JC, Lamy M, Fallat RJ, Koeniger E, Yaeger EM, Whitmer G (1976) Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. J Thorac Cardiovasc Surg 71:64–71
Martin C, Papazian L, Payan MJ, Saux P, Gouin F (1995) Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. [see comments]. Chest 107:196–200
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet J-F, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. New Engl J Med 346:1281–1286
Raghu G, Masta S, Meyers D, Narayanan AS (1989) Collagen synthesis by normal and fibrotic human lung fibroblasts and the effect of transforming growth factor-beta. Am Rev Respir Dis 140:95–100
Budinger G, Chandel N, Donnelley H, Eisenbart J, Oberoi M, Jain M (2004) Active transforming growth factor-ß1 contributes to procollagen I promoter activation in early lung injury. Am J Respir Crit Care Med 169:A350
Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW (1999) TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int 56:1710–1720
Farjanel J, Hartmann DJ, Guidet B, Luquel L, Offenstadt G (1993) Four markers of collagen metabolism as possible indicators of disease in the adult respiratory distress syndrome. Am Rev Respir Dis 147:1091–1099
Armstrong L, Thickett DR, Mansell JP, Ionescu M, Hoyle E, Billinghurst RC, Poole AR, Millar AB (1999) Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med 160:1910–1915
Pugin J, Verghese G, Widmer MC, Matthay MA (1999) The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. [see comments]. Crit Care Med 27:304–312
Madtes DK, Rubenfeld G, Klima LD, Milberg JA, Steinberg KP, Martin TR, Raghu G, Hudson LD, Clark JG (1998) Elevated transforming growth factor-alpha levels in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 158:424–430
Fahy RJ, Lichtenberger F, McKeegan CB, Nuovo GJ, Marsh CB, Wewers MD (2003) The acute respiratory distress syndrome: a role for transforming growth factor-beta 1. Am J Respir Cell Mol Biol 28:499–503
Nerlich AG, Nerlich ML, Muller PK (1987) Pattern of collagen types and molecular structure of collagen in acute post-traumatic pulmonary fibrosis. Thorax 42:863–869
Zhao Y, Young SL, McIntosh JC, Steele MP, Silbajoris R (2000) Ontogeny and localization of TGF-beta type I receptor expression during lung development. Am J Physiol Lung Cell Mol Physiol 278:L1231–1239
Broekelmann TJ, Limper AH, Colby TV, McDonald JA (1991) Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A 88:6642–6646
Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH (1991) Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 5:155–162
Hiwatari N, Shimura S, Yamauchi K, Nara M, Hida W, Shirato K (1997) Significance of elevated procollagen-III-peptide and transforming growth factor-beta levels of bronchoalveolar lavage fluids from idiopathic pulmonary fibrosis patients. Tohoku J Exp Med 181:285–295
Salez F, Gosset P, Copin MC, Lamblin Degros C, Tonnel AB, Wallaert B (1998) Transforming growth factor-beta1 in sarcoidosis. Eur Respir J 12:913–919
Kotecha S, Wangoo A, Silverman M, Shaw RJ (1996) Increase in the concentration of transforming growth factor beta-1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity. J Pedriatr 128:464–469
Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude OP, Laurent GJ (1998) Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. Biochem J 333 (Pt 1):121–127
Pardes JB, Takagi H, Martin TA, Ochoa MS, Falanga V (1995) Decreased levels of alpha 1(I) procollagen mRNA in dermal fibroblasts grown on fibrin gels and in response to fibrinopeptide B. J Cell Physiol 162:9–14
Krupsky M, Fine A, Kuang PP, Berk JL, Goldstein RH (1996) Regulation of type I collagen production by insulin and transforming growth factor-beta in human lung fibroblasts. Connect Tissue Res 34:53–62
Telasky C, Tredget EE, Shen Q, Khorramizadeh MR, Iwashina T, Scott PG, Ghahary A (1998) IFN-alpha2b suppresses the fibrogenic effects of insulin-like growth factor-1 in dermal fibroblasts. J Interferon Cytokine Res 18:571–577
Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM (2000) Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 292:988–994
Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B (1998) IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol 10:1421–1433
Last JA, Siefkin AD, Reiser KM (1983) Type I collagen content is increased in lungs of patients with adult respiratory distress syndrome. Thorax 38:364–368
Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, Nagae H, Hudson LD, Martin TR (1999) Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 160:1843–1850
Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR (1999) Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol 163:2217–2225
Ware LB, Matthay MA (2001) Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1376–1383
Willis BC, Kim KJ, Li X, Liebler J, Crandall ED, Borok Z (2003) Modulation of ion conductance and active transport by TGF-{beta}1 in alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 285:L1192–L1200
Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D (2001) TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107:1537–1544
Fraslon C, Lacaze-Masmonteil T, Zupan V, Chailley-Heu B, Bourbon JR (1993) Fetal rat lung type II cell differentiation in serum-free isolated cell culture: modulation and inhibition. Am J Physiol 264:L504–516
Hurst VI, Goldberg PL, Minnear FL, Heimark RL, Vincent PA (1999) Rearrangement of adherens junctions by transforming growth factor-beta1: role of contraction. Am J Physiol 276:L582–595
Das SK, White AC, Fanburg BL (1992) Modulation of transforming growth factor-beta 1 antiproliferative effects on endothelial cells by cysteine, cystine and N-acetylcysteine. J Clin Invest 90:1649–1656
Acknowledgements
We wish to thank J. Iasha Sznajder and Lewis Smith for critical review of the data and manuscript and Borko Jovanovic for statistical support. Dr. Budinger was supported by the grant NHLBI K08HL067835, American Lung Association, Crane Asthma Center; Dr. Chandel was supported by the grants NHLBI R01GM060472 and P01HL071643, and Crane Asthma Center; and Dr. Jain by the grant NHLBI/NCRR 1K12RR017707–02, American Lung Association, Northwestern Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Budinger, G.R.S., Chandel, N.S., Donnelly, H.K. et al. Active transforming growth factor-β1 activates the procollagen I promoter in patients with acute lung injury. Intensive Care Med 31, 121–128 (2005). https://doi.org/10.1007/s00134-004-2503-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2503-2