Abstract
Objective
To assess noise exposure during noninvasive ventilation (NIV) with different types of interface (helmet, nasal, and facial masks).
Subjects and methods
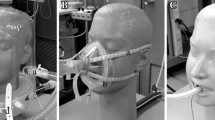
Ten “naive” healthy volunteers underwent NIV at pressure support levels of 10 and 15 cmH2O with: (a) helmet, (b) helmet equipped with HME filters at the junctions between the helmet and the inspiratory and expiratory branches of the respiratory circuit, (c) nasal mask, and (d) facial mask. Noise intensity was assessed with a sound level meter by placing a microphone near the right ear. Noise intensity and degree of discomfort were also assessed subjectively with a visual analogue scale.
Results
Inside the helmet noise exceeded 100 dB. Noise intensity was poorly affected by pressure support level and unaffected by the presence of HME filters. During NIV with nasal or facial masks the noise did not exceed 70 dB (i.e., noise was not louder than the usual noise background in ICU). Subjective evaluation of noise intensity mirrored objective measurements; however, the presence of HME filters was associated with the feeling of less noise inside the helmet. The discomfort associated with the helmet did not significantly differ from that associated with the masks.
Conclusions
NIV helmet is associated with significantly greater noise than nasal and facial masks, but is as comfortable as masks, at least in the short term. Medium- and long-term exposure to loud noise may potentially impair ear function and increase the patient’s discomfort.
Similar content being viewed by others
Introduction
Early application of noninvasive ventilation (NIV) to patients affected by acute respiratory failure proved to be physiologically effective and to prevent the need for intubation, especially in patients affected by chronic obstructive pulmonary disease exacerbations, cardiogenic pulmonary edema, and immunosuppression [1, 2]. In addition, NIV may offer a lower risk of ventilator-associated pneumonia [3, 4, 5, 6] and an easier weaning from mechanical ventilation in comparison with conventional mechanical ventilation through an endotracheal tube [7]. In spite of these advantages the use of NIV is often precluded by the patient’s poor acceptance. Patients treated with NIV cannot receive heavy sedation, and discomfort is a major cause of NIV failure. The choice of the patient/ventilator interface is often critical in this regard, and the solution adopted should fit patient’s features, since facial and nasal masks and more recently helmets differ substantially in terms of dead space, risk of claustrophobia, risk of pressure sores, amount of leaks, and the ability to communicate, assume oral intake, and expectorate [8].
Although it is now clear that loud sounds can contribute to patient discomfort during the ICU stay [9, 10, 11], noise exposure during NIV may be underestimated among the factors that influence patient well-being. While personally experiencing NIV with a helmet, we felt that the noise exceeded the usual ICU background noise, potentially increasing patient discomfort and causing sleep disruption. The aim of this study was therefore to compare the level of noise to which subjects are exposed during NIV with a helmet, a nasal mask, and a facial mask.
Methods
After obtaining local ethics committee approval and informed consent from participants, ten “naive” healthy subjects (aged 23–49 years, median 27; eight men, two women) were included in this study. Criteria of exclusion were: (a) medical history or physical examination positive for ear, nose, or trout diseases, and (b) absence of knowledge or previous experience of NIV. The study was performed in the research laboratory of the postoperative intensive care unit of a University hospital. In a quiet room subjects underwent NIV with a Servo 300 Ventilator (Siemens-Elema, Sweden) in pressure support (PS) modality; inspired fraction of oxygen was 0.21 and positive end-expiratory pressure was 5 cmH2O. Three interfaces were applied in four random sequences; each was tested for 15 min at a PS level of 10 cmH2O and for 15 min at a PS level of 15 cmH2O. The interfaces were:
-
A NIV helmet (Starmed, Mirandola, Italy) of the appropriate size. A breathing circuit with inspiratory and expiratory lines (Tyco, Mirandola, Italy) with an internal diameter of 2 cm and a length of 100 cm was employed to connect the helmet to the ventilator.
-
As above, but a heat and moisture exchanger (HME) filter (Hygrobac antimicrobial filter/HME, Mallinkrodt, Mirandola, Italy) was applied at both junctions between the helmet and the respiratory circuit. The filters were inserted to try to decrease the noise caused by the gas that flowed through the circuit.
-
A facial mask (Tyco, Mirandola, Italy). A breathing circuit equal to that employed with the helmet was used. No HME filter was applied.
-
A nasal mask (Comfortgel, Respironics Deutschland, Germany). A breathing circuit equal to that employed with the helmet was used. No HME filter was applied.
The intensity of the noise to which the subjects were exposed was measured in decibel with a sound level meter TES1350A (TES Electrical Electronic, USA) and the noise intensity perceived by the subjects was assessed by a visual analogue scale (VAS). The sound level meter had a measuring range between 35 and 130 dB (measurements were made by selecting the high range between approx. 65 and 130 dB or the low range between approx. 35 and 80 dB), a frequency range between 31.5 and 8KHz, and a display with 0.1dB steps on a four-digit liquid crystal display. Since the human ear does not respond equally to all frequencies but is much more sensitive to sounds of about 1–4 kHz, measurements of sound intensity are usually performed with filters to better reflect the frequency response of the human ear. A-weighting filters are more commonly utilized; however, in this study measurements were performed on the C scale, i.e., using a C-weighting filter, which is practically linear over several octaves and is more suitable than A-scale for subjective measurements at very high sound levels.
The microphone was fixed with plaster on the anterior surface of the right tragus corresponding to the gas inlet to the helmet. A thin electrical wire connected the microphone to the sound level meter so that there was no problem of airtightness during measures performed within the helmet. While the function “max. hold” was selected, the sound level meter displayed the maximal sound intensity registered. Two intervals of 1 min were taken into account for each measurement, and the mean between the two maximal sound intensities was registered. Prior to each session of the study, a calibration was made at 94 dB. We also assessed the noise background in the ICU at patients’ bedside in absence of peak sounds (respiratory or monitor alarms, talking). The noise perceived by the subjects and the degree of discomfort caused by NIV were both determined at the end of the 15-min interval at a PS level of 15 cmH2O with VAS ranging from 0 (absence of noise/no discomfort) to 10 (unbearable noise/unbearable discomfort). The subjects were asked the questions: “How do you estimate the noise you were exposed to during the procedure?” and “How do you estimate the degree of discomfort during the procedure?” Such evaluation was performed only after the 15-min interval at a PS level of 15 cmH2O to avoid a potential bias due to the nonrandomized PS sequence from 10 to 15 cmH2O.
Sound level meter measurements were analyzed by two-way analysis of variance for repeated measurements and are reported as means and standard deviations. VAS scores were analyzed by Friedman’s analysis of variance and are reported as medians and 10th–90th percentiles. The Student-Newman-Keuls test was used for post-hoc analysis of sound level meter measurements and of VAS scores.
Results
The sound intensities registered during the study ranged between 60 and 110 dB, which means that the highest sound intensities were about 104 times larger than the lowest ones. Interfaces affected the noise level associated with NIV significantly (analysis of variance, p<0.001), while the difference related to the level of PS (10 or 15 cmH2O) did not reach statistical significance (p=0.0666). Values are shown in Table 1.
The noise inside the helmet always exceeded 100 dB. The effect of positioning the microphone near to the left ear (gas outlet) instead of to the right ear (gas inlet) was tested initially, but no noticeable difference was pointed out so, and therefore both ears were exposed to similar noise levels regardless of the side, near to the gas inlet or opposite to. The main source of the noise appeared to be the gas flowing through the circuit since the intensity of the noise measured at the extremity of the inspiratory branch of the circuit while it was disconnected from the helmet equaled that previously measured inside the helmet. The same values were observed when placing the captor inside the opened nasal and facial masks. The presence of filters at the junctions between the circuit and the helmet did not affect noise intensity relevantly, but might have slightly attenuated the noise at a PS level of 15 cmH2O. In this regard, post-hoc comparisons (see Table 1) showed that the noise inside the helmet without filters increased when moving from a PS level of 10 cmH2O to a PS level of 15 (p=0.0018) and was louder than the noise at the same PS level of 15 cmH2O in presence of filters (p=0.0009).
The sound intensity registered during NIV with facial or nasal mask was significantly lower and scarcely exceeded 60 dB regardless of the level of PS (10 or 15 cmH2O). This level of noise did not exceed ICU background noise, which ranged between 55 and 65 dB.
Also the subjective perception of noise during NIV was affected by the ventilator-patient interface (Friedman analysis, p≤0.001; Fig. 1). Post-hoc analysis demonstrated that during NIV with helmets without filters, the volunteers scored VAS values significantly higher than during NIV with facial or nasal masks (p≤0.05) while no significant difference was observed between facial and nasal masks. All subjects judged that noise inside helmets was lower in presence of HME filters than in absence, but the VAS difference did not reach statistical significance.
Degree of noise caused by the helmet, facial mask, and nasal mask at a PS level of 15 cmH2O, evaluated by VAS (0=no noise; 10=unbearable noise). Friedman analysis: comparison between interfaces p≤0.001. Significant post-hoc comparisons (p≤0.05): helmet, no HME filters vs. facial mask; helmet, no HME filters vs. nasal mask
The degree of discomfort caused by NIV with helmet, nasal, and facial masks are shown in Fig. 2. Helmet and nasal mask were better tolerated than facial mask by most subjects; however, differences did not reach statistical significance.
Discussion
Helmets have been initially used for continuous positive airway pressure and only recently have been proposed as patient-ventilator interfaces for NIV. Helmets offer some undoubted advantages in comparison with other interfaces, such as the ability to communicate, drink, and read without the feeling of uncomfortable pressure on the face; conversely, they may lead to dysynchrony between patients and ventilators [12]. A recent study comparing the effectiveness of helmets and facial masks for delivering NIV mentioned noise as a possible negative effect of this interface [13]. A short trial of NIV is indeed sufficient to perceive the loud noise that spreads inside the helmet. In this study such noise was quantified to evaluate the potential risk for the patient and possible remedies.
The intensity of noise inside the helmet during NIV was about 105 dB and was mostly caused by the turbulent gas flow through the respiratory circuit. By contrast, the noise perceived during NIV with nasal or facial masks was probably mostly caused by the ventilator and was about 65 dB, not different from the background noise that was measured at patients’ bedside in our ICU and that was reported by other authors [14]. Since the noise caused by gas flow may theoretically diffuse to the middle ear through the bone during NIV with masks, we also assessed the subjective perception of sound intensity. VAS values confirmed the difference determined by sound level meter measurements between the noise of helmets and masks. Such difference, about 40 dB, may falsely appear small due to the unit of measurement utilized (see Appendix).
Loud noise may damage the ear. According to the equal energy hypothesis, the hearing loss from a given exposure is proportional to the total energy of the exposure [15]. On this basis intensity and duration of exposure are equally important, and an exposure to 90 dB for 8 h is the same as 105 dB for 15 min, since an increase of 3 dB is equivalent to a doubling of the noise dose. Damage of hair cells of the inner ear has been reported following prolonged exposure to noise over 90 dB [16]. Damage may result in a permanent hearing loss, but more frequently causes a temporary auditory threshold shift (TTS), which may require days to recover if caused by prolonged noise exposure (days) at 100 dB [17]. Tinnitus, usually transitory, may also occur after noise exposure, particularly in patients with preexisting hearing loss [18]. It is not clear whether impulse noise is more or less damaging than steady state noise [17]; however, applying sounds at 100 dB at a frequency of 6/min for periods as long as 1–3 days resulted in TTS and time to recover variable according to the percentage of each cycle in which the noise was present. When the sound was applied for only 30% of each cycle (a condition mirroring the exposure to noise inside the helmet) TTS was mild and recovery was quick [17]. Finally, a physiological mechanism to protect the ear against impulse noise is based on the contraction of stapedius muscle, which tightens the ossicular chain; in the presence of unilateral lower motor neuron facial palsy, the paralysis of stapedius muscle results in a more pronounced TTS on the paralyzed side [19]. The effectiveness of stapedius muscle contraction may be decreased during NIV with helmet. The occurrence of stapedius muscle fatigue has been recently hypothesized to explain the increase in acoustic compliance of tympanic membrane observed after 1 h of NIV with helmet in healthy volunteers [20]. Such fatigue may occur because of the periodic deformation of the tympanic membrane caused by the inspiratory increase in pressure inside the helmet.
The excess of noise inside the helmet may have some detrimental effects on patient wellness. Although the National Institute for Occupational Safety and Health (NIOSH) in the United States recommends that noise intensity in hospitals should not exceed 40 dB during the day and 35 dB at night [21], the ICU environment is usually far more noisy, having a background noise around 65 dB [14, 22] and peak sound levels that exceed 80 dB. Staff conversations, monitor alarms, ventilator alarms, and telephones account for almost all of peak sounds [14, 23]. Such noise intensity has been regarded in the past as a major cause of sleep arousals in critically ill patients, and a very strong correlation was observed between sleep arousals and occurrence of peak sounds louder than 80 dB [24]. However, more recent studies have reported that sleep disruption in ICU is multifactorial, and that noise is responsible for only a limited proportion of arousals and awakenings [10, 11]. The noise to which patients are exposed within the helmet is much more intense than ICU background noise and than most peak sounds in ICUs and may potentially cause more sleep disruption. Fortunately, this is not necessarily true, since the noise inside the helmet is intermittent, but regular. Furthermore human ear perception of sound intensity is not linear and louder sounds are underestimated so that subjective perception may be less pronounced and may disrupt patient’s sleep less than it might be expected. Finally, patients undergoing NIV with helmets seldom complain of noise as a major cause of discomfort.
Noise may also impair patient’s contact with the environment by masking sounds and hindering conversation, and this inconvenience may add to the physical barrier that the helmet constitutes. It is not surprising that in this study these factors did not affect the degree of discomfort caused by helmet, which was not higher than that caused by masks, since the evaluation was performed in a quiet room after a short-term application in healthy subjects. Further data are needed to compare helmet and mask acceptance during long-term NIV application in patients suffering dyspnea, pain, anxiety, although clinical studies suggest an increased level of tolerance with the helmet both in hypoxemic [2] and hypercapnic [12] acute respiratory failure patients.
Noise exposure during NIV with helmets may be attenuated by some devices. HME filters did not affect noise intensity measured by the sound level meter, but decreased the noise perceived by the subjects. This effect may be hypothetically explained by a selective effect on the sound frequencies to which the human ear is more sensitive and may effectively decrease patient’s discomfort caused by the noise inside the helmet. Other devices include earplugs and sound traps. Ear plugs may be effective against sleep disruption but may also make the contact with the environment more difficult. Conversely, adding sound traps to the inspiratory branch of the respiratory circuit may potentially limit noise inside the helmet without major inconvenience.
In conclusion, NIV with helmet expose patients to high-intensity noise, which may increase patient’s discomfort and affect ear function. Although the subjects included in this study judged NIV with the helmet as comfortable as with nasal or facial masks, noise may decrease the acceptance of NIV with helmet during long-term treatments, particularly in patients suffering dyspnea, pain, anxiety. Devices as HME filters, ear plugs, and sound traps may effectively decrease the discomfort caused by noise inside the helmet.
References
Brochard L, Mancebo J, Elliott MW (2002) Noninvasive ventilation for acute respiratory failure. Eur Respir J 19:712–721
Antonelli M, Pennisi MA, Conti G (2003) New advances in the use of noninvasive ventilation for acute hypoxemic respiratory failure. Eur Respir J 22 Suppl 42:65s-71 s
Carlucci A, Richard JC, Wysocki M, Lepage E, Brochard L (2001) Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med 163:874–880
Nourdine K, Combes P, Carton M-J, Beuret P, Cannamela A, Ducreux J-C (1999) Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med 25:567–573
Girou E, Schortgen F, Delclaux C, Brun-Buisson C, Blot F, Lefort Y, Lemaire F, Brochard L (2000) Association of non-invasive ventilation with nosocomial infections and survival in critically ill patients. JAMA 284:2361–2367
Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, Gasparetto A, Meduri GU (1998) A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 339:429–435
Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, Brigada P, Fracchia C, Rubini F (1998) Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 128:721–728
Schönhofer B, Sortor-Leger S (2002) Equipment needs for noninvasive mechanical ventilation. Eur Respir J 20:1029–1036
Freedman NS, Kotzer N, Schwab RJ (1999) Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med 159 (4 Pt 1):1155–1162
Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ (2001) Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med 163:451–457
Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ (2003) Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med 167:708–715
Antonelli M, Pennisi MA, Pelosi P, Gregoretti C, Squadrone V, Rocco M, Cecchini L, Chiumello D, Severgnini P, Proietti R, Navalesi P, Conti G (2004) Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease: a feasibility study. Anesthesiology 100:16–24
Chiumello D, Pelosi P, Carlesso E, Severgnini P, Aspesi M, Gamberoni C, Antonelli M, Conti G, Chiaranda M, Gattinoni L (2003) Noninvasive positive pressure ventilation delivered by helmet vs. standard face mask. Intensive Care Med 29:1671–1679
Allaouchiche B, Duflo F, Debon R, Bergeret A, Chassard D (2002) Noise in the postanaesthesia care unit. Br J Anaesth 88:369–373
Goulios H, Robertson D (1983) Noise-induced cochlear damage assessed using electrophysiological and morphological criteria: an examination of the equal energy principal. Hear Res 11:237–242
Ward WD (1979) General auditory effects of noise. Otolaryngol Clin North Am 12:473–492
Patuzzi R (1998) Exponential onset and recovery of temporary threshold shift after loud sound: evidence for long-term inactivation of mechano-electrical transduction channels. Hear Res 125:17–38
Nott MR, West PDB (2003) Orthopedic theatre noise: a potential hazard to patients. Anaesthesia 58:784–787
Zakrisson J-E, Borg E, Diamant H, Møller AR (1975) Auditory fatigue in patients with stapedius muscle paralysis. Acta Otolaryngol (Stockh) 79:228–232
Cavaliere F, Masieri S, Conti G, Antonelli M, Pennisi MA, Filipo R, Proietti R (2003) Effects of non-invasive ventilation on middle ear function in healthy volunteers. Intensive Care Med 29:611–614
National Institute for Occupational Safety and Health (1998) Occupational noise exposure. Revised criteria. National Institute for Occupational Safety and Health, Cincinnati
Meyer TJ, Eveloff S, Bauer M, Schwartz W, Hill N, Millman R (1994) Adverse environmental condition in the respiratory and medical ICU settings. Chest 105:1211–1216
Kahn DM, Cook TE, Carlisle CC, Nelson DL, Kramer NR, Millman RP (1998) Identification and modification of environmental noise in an ICU setting. Chest 114:535–540
Aaron JN, Carlisle CC, Carskadon MA, Meyer TJ, Hill NS, Millman RP (1996) Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep 19:707–710
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The dB is a logarithmic unit used to measure not only sound intensity but also other variables, such as power, voltage, or intensity in electronics, signals, and communications. The dB is the logarithm to base 10 of the ratio between the value measured and a reference value: dB=10 log (S2/S1). Consequently the ratio between two sounds can be easily calculated from the difference in dB: S2/S1=10(dB2−dB1)/10. For example, a difference of 60 dB corresponds to a ratio of 106. The reference value usually chosen for sound intensity measurements in air is 20 kPa. This intensity corresponds to the limit of sensitivity of the human ear in its most sensitive range of frequency. According to the formula previously reported, a sound sharing the same intensity with the reference sound is 0 dB, while negative dB values correspond to sounds with lower intensity.
Rights and permissions
About this article
Cite this article
Cavaliere, F., Conti, G., Costa, R. et al. Noise exposure during noninvasive ventilation with a helmet, a nasal mask, and a facial mask. Intensive Care Med 30, 1755–1760 (2004). https://doi.org/10.1007/s00134-004-2347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2347-9