Abstract
Objective
To assess the effects of the potassium ATP (KATP) channel blocker HMR1402 (HMR) on systemic and hepato-splanchnic hemodynamics, oxygen exchange and metabolism during hyperdynamic porcine endotoxemia.
Design
Prospective, randomized, controlled study with repeated measures.
Setting
Animal laboratory.
Subjects
Eighteen pigs allocated to receive endotoxin alone (control group, CON, n=10) or endotoxin and HMR (6 mg/kg h−1, n=8).
Interventions
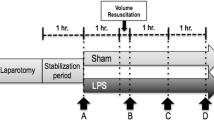
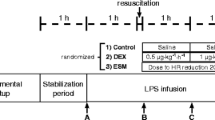
Anesthetized, mechanically ventilated, and instrumented pigs receiving continuous i.v. endotoxin were resuscitated with hetastarch to maintain mean arterial pressure (MAP) >60 mmHg. Twelve hours after starting the endotoxin infusion, they received HMR or its vehicle for another 12 h.
Results
HMR transiently increased MAP by about 15 mmHg, but this effect was only present during the first 1 h of infusion. The HMR decreased cardiac output due to a fall in heart rate, and thereby reduced liver blood flow. While liver O2 delivery and uptake remained unchanged, HMR induced hyperlactatemia [from 1.5 (1.1; 2.0), 1.4 (1.2; 1.8), and 1.2 (0.8; 2.0) to 3.1 (1.4; 3.2), 3.2 (1.6; 6.5), and 3.0 (1.0; 5.5) mmol/l in the arterial, portal and hepatic venous samples, respectively] and further increased arterial [from 8 (3; 13) to 23 (11; 57); p<0.05], portal [from 9 (4; 14) to 23 (14; 39); p<0.05] and hepatic vein [from 7 (0; 15) to 30 (8; 174), p<0.05] lactate/pyruvate ratios indicating impaired cytosolic redox state.
Conclusion
The short-term beneficial hemodynamic effects of KATP channel blockers have to be weighted with the detrimental effect on mitochondrial respiration.




Similar content being viewed by others
References
Landry DW, Oliver JA (2001) The pathogenesis of vasodilatory shock. N Engl J Med 345:588–595
Hall S, Turcato S, Clapp L (1996) Abnormal activation of K+ channels underlies relaxation to bacterial lipolysaccharide in rat aorta. Biochem Biophys Res Commun 224:184–190
Chen SJ, Wu CC, Yang SN, Lin CI, Yen MH (2000) Hyperpolarization contributes to vascular hyporeactivity in rats with lipolysaccharide-induced endotoxic shock. Life Sci 68:659–668
Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, Downey JM, Benoit JN (2002) Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol 97:365–373
Macarthur H, Couri DM, Wilken GH, Westfall TC, Lechner AJ, Matuschak GM, Chen Z, Salvemini D (2003) Modulation of serum cytokine levels by a novel superoxide dismuatse mimetic, M40401, in an Escherichia coli model of septic shock: correlation with preserved circulating catecholamines. Crit Care Med 31:237–245
Wilson AJ, Clapp LH (2002) The molecular site of action of K(ATP) channel inhibitors determines their ability to inhibit iNOS-mediated relaxation in rat aorta. Cardiovasc Res 56:154–163
Landry DW, Oliver JA (1992) The ATP-sensitive K+ channels mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J Clin Invest 89:2071–2074
Vanelli G, Sabah NA, Hussain SN, Aguggini G (1995) Glibenclamide, a blocker of ATP-sensitive potassium channels, reverses endotoxin-induced hypotension in pig. Exp Physiol 80:167–170
Wu CC, Thiemermann C, Vane JR (1995) Glibenclamide-induced inhibition of the expression of inductible nitric oxide synthase in cultured macrophages and in the anaesthetized rat. Br J Pharmacol 114:1273–1281
Vanelli G, Hussain SN, Dimori M, Aguggini G (1997) Cardiovascular responses to glibenclamide during endotoxaemia in the pig. Vet Res Commun 21:187–200
Sorrentino R, d’Emmanuele di Villa Bianca R, Lippolis L, Sorrentino L, Autore G, Pinto A (1999) Involvement of ATP-sensitive potassium channels in a model of delayed vascular hyporeactivity induced by lipopolysaccharide in rats. Br J Pharmacol 127:1447–1453
Preiser JC, Zhang H, Debelle F, Fesler P, Abdel Kafi S, Naeije R, Vincent JL (2003) Hemodynamic effects of glibenclamide during endotoxemia: contrasting findings in vitro versus in vivo. Shock 19:223–228
Ismail JA, McDonough KH (2001) The role of K+ ATP channels in the control of pre- and post-ischemic left ventricular developed pressure in septic rat hearts. Can J Physiol Pharmacol 79:213–219
Wikstöm BG, Ronquist G, Waldenström A (1996) Glyburide enhancement of lactate production in ischemic heart is modified by preconditioning: an in vivo experimental study in pigs by microdialysis technique. J Cardiovasc Pharmacol 27:622–628
Fink MP, Heard SO (1990) Laboratory models of sepsis and septic shock. J Surg Res 49:186–196
Billman GE, Houle MS, Gerlach U, Englers HC, Goegelein HE (2000) The cardioselective ATP-sensitive potassium channel antagonist HMR1402 prevents ischemically induced ventricular fibrillation. Europace 1:B29
Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA (2001) Post-injury multiple organ failure: the role of the gut. Shock 15:1–10
Iványi Z, Hauser B, Pittner A, Asfar P, Vassilev D, Nalos M, Brückner UB, Szabó C, Radermacher P, Fröba G (2003) Systemic and hepato-splanchnic hemodynamic and metabolic effects of the PARP inhibitor PJ34 during hyperdynamic porcine endotoxemia. Shock 19:415–425
Nalos M, Vassilev D, Pittner A, Asfar P, Brückner UB, Schneider EM, Georgieff M, Radermacher P, Fröba G (2003) Sn-mesoporphyrin to inhibit heme oxygenase during long-term hyperdynamic porcine endotoxemia. Shock 19:526–532
Stehr A, Ploner F, Tugtekin I, Matejovic M, Theisen M, Zülke C, Georgieff M, Radermacher P, Jauch K-W (2003) Effect of combining nicotinamide as a PARS-inhibitor with selective iNOS Blockade during porcine endotoxemia. Intensive Care Med 29:995–1002
Pittner A, Nalos M, Asfar P, Yang Y, Ince C, Georgieff M, Brückner UB, Radermacher P, Fröba G (2003) Mechanisms of iNOS inhibition-related improvement of gut mucosal acidosis during hyperdynamic porcine endotoxemia. Intensive Care Med 29:312–316
Dhein S, Pejman P, Krusemann (2000) Effects of the I(KATP) blockers glibenclamide and HMR1883 on cardiac electrophysiology during ischemia and reperfusion. Eur J Pharmacol 398:273–284
Englert HC, Gerlach U, Goegelein H, Hartung J, Heitsch H, Mania D, Scheidler S (2001) Cardioselective K(ATP) channel blockers derived from a new series of m-anisamidoethylbenzenesulfonylthioureas. J Med Chem 44:1085–1098
Krismer AC, Wenzel V, Voelckel W, Witkiewicz M, Strohmenger HU, Raedler C, Lindner KH (2002) Effect of the cardioselective ATP-sensitive potassium channel inhibitor HMR 1883 in a porcine model of cardiopulmonary resuscitation. Resuscitation 53:299–306
Szabó C, Salzman AL (1996) Inhibition of ATP-activated potassium channels exerts pressor effects and improves survival in a rat model of severe hemorrhagic shock. Shock 5:391–394
Salzman AL, Vromen A, Denenberg A, Szabó C (1997) K(ATP)-channel inhibition imporves hemodynamics and cellular energetics in hemorrhagic shock. Am J Physiol 272:H688–H694
Zhao K, Huang X, Liu J, Huang Q, Jin C, Jiang Y, Jin J, Zhao G (2002) New approach to treatment of shock: restitution of vasoreactivity. Shock 18:189–192
Billman GE, Avendano CE, Halliwill JR, Burroughs JM (1993) The effects of the ATP-dependent potassium channel antagonist, glyburide, on coronary blood flow and susceptibility to ventricular fibrillation in unanesthetized dogs. J Cardiovasc Pharmacol 21:197–204
Kamigaki M, Ichihara K, Kohgo Y, AbikoY (1995) Effect of glibenclamide on ischemic canine myocardium with glucose infusion. Eur J Pharmacol 287:121–126
de Jaeger A, Proulx F, Yandza T, Dugas MA, Boeuf B, Manika A, Lacroix J, Lambert M (1998) Markers of cellular dysoxia during orthotopic liver transplantation in pigs. Intensive Care Med 24:268–275
Leverve XM (1999) From tissue perfusion to metabolic marker: assessing organ competition an co-operation in critically ill patients? Intensive Care Med 25:890–892
Levy B, Mansart A, Bollaert PE, Franck P, Mallie JP (2003) Effects of epinephrine and norepinephrine on hemodynamics, oxidative metabolism, and organ energetics in endotoxemic rats. Intensive Care Med 29:292–300
Thomas DW, Gilligan JE, Edwards JB, Edwards RG (1972) Lactic acidosis and osmotic diuresis produced by xylitol infusion. Med J Aust 1:1246–1248
Harkema JM, Chaudry ICH (1992) Magnesium-adenosine triphophate in the treatment of shock, ischemia, and sepsis. Crit Care Med 20:263–275
Acknowledgements
This work was supported by the Centre Hospitalo-Universitaire, Angers, France (P. Asfar), the Deutscher Akademische Austischdienst (Z. Iványi), the Alexander-von-Humboldt-Stiftung (D. Vassilev), the European Society of Intensive Care Medicine (B. Hauser), the Boehringer Ingelheim Fonds (M. Nalos), and Aventis Pharma. HMR1402 was kindly provided by K. Wirth (Aventis Pharma, Germany). We are indebted to W. Siegler, T. Schulz, U. Ehrmann, A. Derr, and M. Miersch for skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Asfar and Z. Iványi equally contributed to this work
Rights and permissions
About this article
Cite this article
Asfar, P., Iványi, Z., Bracht, H. et al. HMR1402, a potassium ATP channel blocker during hyperdynamic porcine endotoxemia: effects on hepato-splanchnic oxygen exchange and metabolism. Intensive Care Med 30, 957–964 (2004). https://doi.org/10.1007/s00134-004-2258-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2258-9