Abstract
Objective
To evaluate the reliability and clinical value of partial noninvasive CO2 (NICO2) rebreathing technique for measuring cardiac output compared with standard thermodilution in a group of intensive care nonpostoperative patients.
Design and setting
Clinical investigation in a university hospital ICU.
Patients
Twelve mechanically ventilated patients with high ( n =6) and low ( n =6) pulmonary shunt fractions.
Measurements and results
Thirty-six paired measurements of cardiac output were carried out with NICO2 and thermodilution in patients ventilated in pressure-support mode and sedated with a sufentanil continuous infusion to obtain a Ramsay score value of 2. The mean cardiac output was: thermodilution 7.27±2.42 l/min; NICO2 6.10±1.66 l/min; r 2was 0.62 and bias −1.2 l/min±1.5. Mean values of cardiac output were similar in the low shunt group (\( {\dot{\text {Q}}} {\text {s}} /\ {\dot{\text {Q}}} {\text {t}} < 20 \)), with r 2=0.90 and a bias of 0.01 l/min±0.4; conversely, in the high pulmonary shunt group (\( {\dot{\text {Q}}} {\text {s}} /\ {\dot{\text {Q}}} > 35\% \)) the mean was 9.32±1.23 l/min with thermodilution and a mean NICO2CO value was 6.97±1.53 l/min, with r 2 of 0.38 and a bias of −2.3 l±1.2 min.
Conclusions
The partial CO2 rebreathing technique is reliable in measuring cardiac output in nonpostoperative critically ill patients affected by diseases causing low levels of pulmonary shunt, but underestimates it in patients with shunt higher than 35%.
Similar content being viewed by others
Introduction
Hemodynamic evaluation is commonly used in critically ill patients to achieve a balance between systemic oxygen delivery and oxygen demand. A number of studies [1, 2, 3] have demonstrated that knowledge of cardiac output (CO) is useful in guiding therapy in anesthesia, intensive care, and emergency department patients. Since its initial description [4] the pulmonary artery catheter has become accepted as a valuable tool for monitoring patient and guiding therapy in ICU. Several complications have been observed with the use of a pulmonary artery catheter [5] including catheter-related infection of the bloodstream [6]. All ICU physicians are therefore interested in the development of accurate, noninvasive methods of CO measurement. In the past 15 years there has been a continuing move to find less invasive techniques to assess CO, including devices based on rebreathing CO2 method. A new device of noninvasive CO monitoring (NICO2-Novametrix Medical System, Conn., USA) using the Fick principle and partial CO2 rebreathing may have a role in the hemodynamic monitoring of critically ill patients following endotracheal intubation. This device has been validated in surgical patients receiving controlled mechanical ventilation during sedation and paralysis.
The purpose of this study was to evaluate the reliability and clinical value of partial CO2 rebreathing technique for measurement of CO (NICO2CO) compared with thermodilution cardiac output technique (TDCO) in critically ill patients admitted to our ICU for different diseases and receiving assisted ventilation in pressure support mode.
Patients and methods
After obtaining the approval of our institutional review board and informed consent from patients’ legal guardians, 36 paired measurements of cardiac output were carried out in 12 stable critically ill patients (two acute myocardial infarctions, two cardiogenic shock, two postoperative respiratory failures, six acute lung injury acute respiratory distress syndrome with sepsis caused by pneumonia). Enrollment criteria were clinical need for hemodynamic monitoring with a Swan Ganz catheter. Exclusion criteria were (a) immediately postoperative phase, (b) central nervous system disorders, (c) chronic obstructive pulmonary disease exacerbation, (d) age under 18 or over 80 years, and (e) severe tricuspid insufficiency.
All patients were ventilated in pressure-support mode, with pressure-support levels ranging from 16 to 20 cmH2O to obtain tidal volumes higher than 7 ml/kg and a respiratory rate less than 25 b/min. No imposed breath was added. They were sedated with sufentanil continuous infusion (0.003–0.005 µg/kg per minute) to obtain a Ramsay score value of 2 [7].
Hemodynamic measurements
Thermodilution cardiac output measurements were carried out approximately every 4 h using a 7.5-F flow directed catheter (Arrow, Arrow International. USA) connected to a monitor (Siemens Elema, Sweden). TDCO was obtained using three 10-ml bolus injections of 5% dextrose water at a temperature of approx. 5–6°C. The averaged values of the three measurements were considered to obtain each cardiac output. We calculated the pulmonary shunt values according to standard formulas: \( {\dot{\text {Q}}} {\text {s}} /\ {\dot{\text {Q}}} {\text {t}} \)=(CcO2−CaO2)/(CcO2−CvO2) (%) at FIO2 =1, where \( {\dot{\text {Q}}} {\text {s}} \) =shunted pulmonary blood flow, \( {\dot{\text {Q}}} {\text {t}} \)=total pulmonary blood flow, CcO2 =pulmonary capillary oxygen content, CaO2 =arterial oxygen content, and CvO2 =venous oxygen content.
Noninvasive measurement of CO was performed with a NICO2 system. This system consists of a disposable device inserted between the ventilator circuit and the endotracheal tube, computer (software version 3.0), and pulse oximeter. The disposable device consists of a rebreathing pneumatically controlled valve that can direct flow through an adjustable rebreathing loop, infrared light absorption CO2 sensor, and air flow sensor. On a breath-by-breath basis CO2 elimination (\( {\dot{\text{V}}} {\text {CO}}_{2} \)) is calculated from the flow and CO2 concentration at the airway opening. Arterial CO2 content is estimated from the PETCO2 and CO2 dissociation curve [8]. The computer cycles every 3 min from the nonbreathing mode (baseline) to a 50-s period in which an additional dead space is included (in the rebreathing loop) to achieve partial CO2 rebreathing.
Pulmonary capillary blood flow, the part of CO that has passed through the ventilated part of the lungs, can be calculated [8] from the differential Fick equation as the differences in V̇CO2 and PETCO2 between the normal and the rebreathing state. Assuming no significant change in pulmonary capillary blood flow during the measurement period, the CO2 Fick equation is: \( {\dot{\text{Q}}}_{{{\text{PCBF}}}} \)=\( \Delta {\dot{\text{V}}} {\text{CO}}_{2} \)/(CvCO2.nonrebr −CaCO2.nonrebr)−(CvCO2.rebr −CaCO2.rebr), where \( {{\dot{\text{Q}}}}_{{{\text{PCBF}}}} \)=pulmonary capillary blood flow ml/min, CaCO2 =alveolar CO2 blood contents ml/ml blood, and CvCO2 =mixed venous CO2 blood contents ml/ml blood. Venous CO2 concentration remains relatively constant during the CO2 rebreathing procedure because of the large size of the CO2 body stores and slow time constant of the CO2 stores relative to the time of rebreathing; therefore the following equation can substitute the previous one: \( {{\dot{\text{Q}}}}_{{{\text{PCBF}}}} \)=\( \Delta {\dot{\text{V}}} {\text{CO}}_{2} \)/ΔCaCO2. Assuming that dead space fraction (Vd/Vt) remains constant during the measurement period, and Δ CaCO2 is proportional to changes in arterial CO2 pressure (PaCO2) and end-tidal CO2 pressure (PETCO2), the following equation can be plotted as: \( {\dot{\text{Q}}}_{{{\text{PCBF}}}} \)=\( \Delta {\dot{\text{V}}} {\text{CO}}_{2} \)/SΔPETCO2 [8]. Δ PETCO2 is the change in PETCO2 between normal breathing and CO2 rebreathing. S is the slope of the CO2 dissociation curve from hemoglobin: S =[1.34 ×(Hb) +18.34]/(1+0.193 ×PaCO2) (mlCO2 −1 blood ×mmHg−1). A shunt correction is then added [8] to the final equation to obtain the total cardiac output This fraction is calculated from FIO2 and PaO2 values entered into the computer; the arterial oxygen saturation determined with a pulse oximeter and the Nunn isoshunt graphs [9].
The NICO2 system (version 3.0) used for this study has been developed for use in patients receiving partial ventilatory support and is based on a 60-s basal time, a 50-s rebreathing time, and 70-s stabilization time. This NICO2 monitor was connected to the patients on their admission to the protocol, and for every TDCO measurement obtained we recorded the most recent NICO2 measurement. We thus obtained 36 paired measurements of cardiac output, three in each patient, over a period ranging from 12 to 20 h. We first analyzed the results of the overall population of patients ( n =12, mean age 56.3±9.7 years) and then divided them into two subgroups, based on pulmonary shunt: \( {\dot{\text{Q}}} {\text{s}} /\ {\dot{\text{Q}}} {\text{t}} \) less than 20% and \( {\dot{\text{Q}}} {\text{s}} /\ {\dot{\text{Q}}} {\text{t}} \) greater than 35% ( n =6, mean age 57.8±9.8 years and n =6, mean age 54.8±10.3 years, respectively).
Statistics
The NICO2CO and TDCO values are expressed as mean ±SD. The correlation between the two methods was determined by linear regression ( r 2). Bland-Altman analysis [10] was used to compare the bias and precision of the two methods.
Results
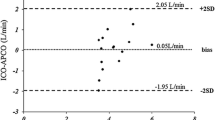
All patients showed stable breathing pattern during the study; measurements were performed in patients with stable respiratory rate and tidal volume variations below 10%, as usually observed in patients receiving moderate sedation; NICO2 measurements were never unobtainable for excessive tidal volume instability. All patients tolerated the rebreathing period. Six patients (\( {\dot{\text{Q}}} {\text{s}} /\ {\dot{\text{Q}}} {\text{t}} \) mean 16±3.5%) had no pulmonary disease: two acute myocardial infarction, two cardiogenic shock, two postoperative acute respiratory failure; six other patients (\( {\dot{\text{Q}}} {\text{s}} /\ {\dot{\text{Q}}} {\text{t}} \) mean 44±6.1%) suffered from acute lung injury/acute respiratory distress syndrome with sepsis caused by pneumonia. The mean cardiac output values of the overall population of 12 patients were as follows: TDCO 7.27±2.42 l/min and NICO2CO 6.10±1.66 l/min, with a moderate correlation ( r 2=0.62) and bias on the Bland-Altman test of 1.2±1.5 l/min (Fig. 1). The values in the low-shunt group were, respectively, 5.22±1.28 and 5.23±1.32 l/min, with a high correlation ( r 2=0.90) and bias of 0.01±0.4 l/min (Fig. 2), and those in the elevated-shunt group were 9.32±1.23 and 6.97±1.53 l/min, with a poor correlation ( r 2=0.38) and a bias of −2.3±1.2 l/min (Fig. 3).
a Scatterplot of cardiac output of the overall group of patients measured by CO2 rebreathing ( NICO 2 CO) and by thermodilution ( TDCO). Solid line Identity. b Difference between and plotted against the average of the two techniques; inner dashed line mean difference (−1.2 l/min); outer dashed lines 95% confidence limits (±1.96 SD, SD=±1.5) of the difference between methods
a Scatterplot of cardiac output of the low-shunt group (Q̇s/Q̇t <20%) measured by CO2 rebreathing ( NICO 2 CO) and by thermodilution ( TDCO). Solid line Identity. b Difference between and plotted against the average of the two techniques; inner dashed line mean difference (0.01 l/min); outer dashed lines 95% confidence limits (±1.96 SD; SD=±0.4) of the difference between methods
a Scatterplot of cardiac output of high-shunt group (Q̇s/Q̇t >35%) measured by CO2 rebreathing ( NICO 2 CO) and thermodilution ( TDCO). Solid line Identity. b Difference between and plotted against the average of the two techniques; inner dashed line mean difference (−2.3 l/min); outer dashed lines 95% confidence limits (±1.96 SD; SD=±1.2) of the difference between methods
Discussion
Thermodilution is the most diffuse technique for CO monitoring, but it requires intravenous catheterization through the heart and into the pulmonary artery. This technique is therefore time consuming and, being a highly invasive procedure, is associated with an increased risk of complications [1, 2]. Designed for use in mechanically ventilated patients during anesthesia or intensive care, the recently introduced NICO2 monitor can measure CO based on changes in respiratory CO2 concentration caused by a brief period of rebreathing. NICO2 monitor is based on a modification of the original Fick method which was based on the principle that oxygen consumption is proportional to the rate of blood pumped by the heart through the lungs, and it can be measured by monitoring gas exchange and invasively sampling blood gases. Specifically, NICO2 uses the Fick principle applied to CO2 produced by the body and eliminated by gas exchange in the lungs This technique, initially called total-rebreathing CO2, became interesting when Gedeon et al. [11] proposed a differential form of the Fick equation, eliminating the need to estimate the CvCO2 and making this technique easier to used. Capek and Roy [12] developed a partial rebreathing technique that allowed regular estimates of CO based on this differential Fick equation but also correcting for alveolar dead space and variations in the CO2 dissociation curve. Initial studies [11, 12] supported the reliability of this method for measurement of CO, even in presence of pulmonary ventilation/perfusion mismatching, although Gedeon and colleagues suggested that the partial CO2 rebreathing technique would not be reliable in measuring CO in patients with pulmonary disease.
The current NICO2 allows, theoretically, a real-time determination of shunt fraction using pulse oxymetry, FIO2, and Noon isoshunt plots. The system is completely noninvasive and easy to use, placing a sensor between the endotracheal tube and the breathing circuit Y piece. Its characteristics propose this technique as a possible alternative to thermodilution for CO determination in ICU patients. Accuracy of the NICO2 monitor has been established on surgical patients mechanically ventilated during anesthesia and the postoperative phase [13, 14, 15]. Most ICU patients are now ventilated with respiratory modes which support spontaneous breathing activity. The updated version of NICO2 software (version 3.0) also allows this technique to be applied in these patients, where minute ventilation could be less stable than during anesthesia.
In this study we tested the procedure in 12 critically ill nonpostoperative patients admitted to our ICU for various diseases and ventilated in pressure support (16–20 cmH2O). We verified the accuracy of this technique in different hemodynamic situations by enrolling patients with and without sepsis-related hemodynamic conditions, dividing them to a group with “low” shunt ( n =6) and another group with “elevated” shunt ( n =6) diseases. Only Tachibana et al. [16] have evaluated CO using partial CO2 rebreathing technique in patients receiving pressure support; however, they enrolled only heavily sedated patients following cardiac surgery who breathed quietly with no variations in tidal volume and relatively normal lung mechanics. This is the first study assessing the reliability of this technique for CO measurements in critically ill patients, provided that a relative stability of respiratory pattern is obtained by moderate level of analgosedation (Ramsay sedation score 2), as required for obtaining reliable values of CO also with thermodilution.
We found a moderate correlation ( r 2=0.62) and a bias of −1.2±1.5 l/min in the overall group of patients but a high correlation ( r 2=0.90) between NICOCO2 and TDCO values in the low-shunt patients. In this subgroup the Bland-Altman analysis revealed a bias of 0.01±0.4 l/min. The correlation coefficient in the elevated-shunt subgroup of patients, on the other hand, was very low. Our data demonstrate a tendency to underestimate the high levels of cardiac output of this partial rebreathing technique. The cause of the low correlation in hyperdynamic patients may be the increased pulmonary shunt present in this subgroup affected by acute lung injury/acute respiratory distress syndrome and sepsis caused by pneumonia. We did not study patients with shunt fraction between 20% and 35% because none of our unselected patients showed these shunt values, but as a general rule the higher the shunt fraction, the greater is the underestimation of CO assessed by the partial CO2 rebreathing technique.
Contrasting data have been reported in recent studies [14, 15, 17, 18] using partial rebreathing methods in patients who have recently undergone major surgery. Van Heerden and colleagues [14] found a moderate correlation ( r 2=0.69) in a group of 12 cardiac surgery patients, and the comparison of the two techniques showed an underestimation at higher values of cardiac output of the noninvasive technique. Binder et al. [17] in a similar group of patients found a bias of 0.05 l/min between the two techniques, proposing the partial rebreathing technique as a valid alternative to thermodilution. Odenstedt and coworkers [15] found a lack of agreement between the shunt estimated by the NICO2 device and the shunt calculated by the standard formula, suggesting that the noninvasive device underestimated because the chosen default value for arteriovenous O2 difference is set at 50 ml/l whereas the measured arteriovenous O2 difference was lower. Nevertheless, they found a good correlation between TDCO and NICO2CO values. Nilsson et al. [18] found a discrepancy between the mean intrapulmonary shunt calculated from the analysis of blood gas and NICO2 computer estimation associated with a lack of agreement between TDCO and NICO2CO in cardiac surgery patients. Gama de Abreu et al. [19] recently published the results of a prospective animal laboratory investigation and clinical trial employing partial rebreathing CO2 technique with V̇CO2 measured breath by breath and thermodilution. The ICU population consisted of eight acute respiratory distress syndrome patients sedated, paralyzed, and ventilated in controlled mode with a mean shunt value of 34.5% The authors concluded that the lack of agreement between the noninvasive technique and the CO estimated by thermodilution was mostly explained by intrapulmonary right to left shunt. In contrast, they found that the partial CO2 rebreathing technique was reliable for measuring the effective nonshunted PCBF, thus representing an useful tool for ventilator adjustments in patients with increased shunt fraction. This aspect has been confirmed by the same authors [20] who evaluated a new device for noninvasive measurement of PCBF by partial CO2 rebreathing in 20 mechanically ventilated patients with acute lung injury. The authors titrated positive end-expiratory pressure levels with the aim of improving PCBF, suggesting that this approach may be useful for guiding adjustments of the respiratory pattern.
Our data show that NICO2CO estimates are reliable only at lower shunt fraction levels, indirectly suggesting that the PCBF measurements are not the source of error but rather the estimation of shunt fraction. The partial CO2 rebreathing technique requires a number of theoretical assumptions that may not apply in ICU patients. Specifically, NICO2 monitoring uses a noninvasive approach to estimate shunt fraction by adapting Nunn’s isoshunt plots. The lack of knowledge regarding hemoglobin P50 could be a major problem in calculating shunt because it affects the real value of hemoglobin saturation. The hemoglobin dissociation curve is in fact shifted to the left by hypothermia, alkalosis, or increased base deficit and to the right by hyperthermia, acidosis, or base excess. The shift to the left or to the right of the hemoglobin dissociation curve change the CcO2−CvO2 difference and subsequently the shunt computation. CcO2 is considered to be equal to PaO2, assuming a particular shape of the hemoglobin dissociation curve. Unfortunately, this assumption is not true in presence of significant P50 alterations. Finally, PaO2 is also affected by CO2 content.
Regarding the possible sources of error in PCBF measurement, a stable CO2 elimination is required, precluding its use in patients with unstable respiratory pattern. In these patients the PETCO2 becomes unstable and impairs the signal-to-noise-ratio. Therefore this technique has always been applied in patients receiving controlled ventilation and heavy sedation. Although PCBF accuracy was not directly evaluated, we found a regular respiratory pattern to be easily obtained even in pressure support mode, suggesting the possibility of obtaining reliable measurements of PCBF. However, the technique itself could cause a bias in the measurements as it involves a moderate increases in PaCO2 (up to 6 mmHg) during the rebreathing period (adding a dead space) while mixed venous PCO2 basically remains unchanged.
In conclusion, our findings in patients ventilated in pressure support mode suggest that the NICO2 software is accurate even in patients with spontaneous breathing activity with moderate variations in breathing pattern and tidal volume. Our results suggest that (a) the partial rebreathing CO2 technique is reliable to measure CO in patients affected by diseases causing low levels of pulmonary shunt in whom because of its simple and rapid application the NICO2 monitor can substitute the standard thermodilution technique, (b) values obtained by the partial rebreathing CO2 technique underestimate CO in patients with elevated pulmonary shunt (although in these patients this technique could be useful for measuring PCBF, guiding PEEP adjustments), and (c) the physician’s decision to use this noninvasive methods for CO or for PCBF evaluation should be based on a careful evaluation of the patient’s clinical picture, chest radiography, and PaO2/FIO2 ratio.
References
Dalen JE, Bone RC (1996) Is it time to pull the pulmonary artery catheter? JAMA 276:916–918
Mimioz O, Rauss A, Rekik N (1994) Pulmonary artery catheterisation in critically ill patients: a prospective analysis of outcome changes associated with catheter-prompted changes in therapy. Crit Care Med 22:573–579
Rivers E, Nguyen B, Havstad S (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Swan HJC, Ganz W, Forester J (1970) Catheterization of the heart in man with use of a flow directed balloon-tipped catheter. N Engl J Med 283:447–451
Polderman KH, Girbes ARJ (2002) Central venous catheter use. I. Mechanical complications. Intensive Care Med 28:1–17
Poldermann KH, Girbes ARJ (2002) Central venous catheter use. II. Infectious complications. Intensive Care Med 28:18–28
Ramsay MAE, Savage TM, Simpson BRJ (1974) Controlled sedation with alphaxalone-alphadolone. BMJ 2:656–659
Jaffe MB (1999) Partial CO2 rebreathing cardiac output operating principle of the NICO system. J Clin Monit Comput 15:387–401
Nunn JF (1993) Nunn’s applied respiratory physiology, 4th edn. Butterworth, Oxford
Bland MJ, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet I:307–310
Gedeon A, Forstund L, Hedenstierna G, Romano E (1980) A new method for non invasive bedside determination of pulmonary blood flow. Med Biol Eng Comput 18:411–418
Capek JM, Roy RJ (1988) Non invasive measurement of cardiac output using partial CO2 rebreathing. IEEE Trans Biomed Eng 35:653–661
Osterlund B, Gedeon A, Krill P, Johansson G (1995) A new method of using gas exchange measurements for the non-invasive determination of cardiac output: clinical experience in adults following cardiac surgery. Acta Anaesthesiol Scand 39:727–732
Van Heerden PV, Baker S, Lim SI, Weidman C, Bulsara M (2000) Clinical evaluation of the non-invasive cardiac output (NICO) monitor in the intensive care unit. Anaesth Intensive Care 28:427–430
Odenstedt H, Stenquist O, Lundin S (2002) Clinical evaluation of partial CO2 rebreathing technique for cardiac output monitoring in critically ill patients. Acta Anaesthesiol Scand 46:152–159
Tachibana K, Imanaka H, Miyano H, Takeuchi M, Kumon K, Nishimura M (2002) Effect of ventilatory settings on accuracy of cardiac output measurement using partial CO2 rebreathing Anesthesiology 96:96–102
Binder JC, Parkin WG (2001) Non-invasive cardiac output determination: comparison of a new partial-rebreathing technique with thermodilution. Anaesth Intensive Care 29:19–23
Nilsson LB, Eldrup N, Berthelsen PG (2001) Lack of agreement between thermodilution and carbon dioxide rebreathing cardiac output. Acta Anaesthesiol Scand 45:680–685
Gama de Abreu M, Quintel M, Ragaller M, Albrecht DM (1997) Partial carbon dioxide rebreathing: a reliable technique for non-invasive measurement of non-shunted pulmonary capillary blood flow. Crit Care Med 25:675–683
Gama de Abreu M, Geiger S, Winkler T, Ragaller M, Pfeiffer T, Leutheuser D, Albrecht DM (2002) Evaluation of a new device for non invasive measurement of non shunted pulmonary capillary blood flow in patients with acute lung injury. Intensive Care Med 28:318–323
Acknowledgements
The authors thank Dr. Alan Pillinger for the careful language revision of the manuscript. This study was funded by an independent research grant from the Department of Anesthesia and Intensive care of the University of Rome La Sapienza.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocco, M., Spadetta, G., Morelli, A. et al. A comparative evaluation of thermodilution and partial CO2 rebreathing techniques for cardiac output assessment in critically ill patients during assisted ventilation. Intensive Care Med 30, 82–87 (2004). https://doi.org/10.1007/s00134-003-2069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2069-4