Abstract
Objective
The inherent properties of an invading bacterium may influence the cytokine profile that is ultimately produced. We determined the alterations in proinflammatory (TNF-α, IL-1, and IL-6) and anti-inflammatory cytokine (IL-10) expressions in lung tissues within the first 48 h after infection in mice with pneumonia induced by direct intratracheal inoculation of five different pneumococcal strains.
Design
Experimental murine model of Streptococcus pneumoniae pneumonia.
Subjects
Female BALB/cby mice aged 8–10 weeks.
Interventions
Five S. pneumoniae clinical isolates were used in this study. The strains included two serotype 3 strains (P4241 and P30606), two serotype 6 strains (P26772 and P23477), and one serotype 19 strain (P15986). The trachea of anesthetized animals was cannulated via the mouth with a blunt needle, and 50 µl bacterial suspension of two different inocula (their respective 100% lethal inoculum and the same 105 CFU/mouse inoculum of S. pneumoniae strains) were instillated. At predetermined times after pneumococcal infection, i.e., time 0 (preinfection) and 2, 4, 6, 12, 24, and 48 h postinfection in experimental groups, lung tissues were sampled from groups of three mice to quantify lung pro- and anti-inflammatory mediators. The experiments were repeated at least three times.
Results
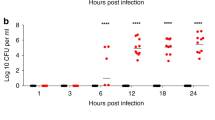
Pneumonia induced by five different pneumococcal isolates resulted in pronounced differences in the local pro- and anti-inflammatory profiles. For example, with a 100% lethal inoculum of S. pneumoniae, the extent and timing of TNF-α expression varied greatly among strains, ranging from 2,643 to 10,022 pg/g and from 4 to 48 h, respectively. Moreover, TNF-α productions within 48 h postinfection measured by the 48 h area under the curve were differed significantly, ranging from 59,700 to 275,825. These different profiles were not serotype dependent. Comparable results were obtained when IL-1, IL-6, and IL-10 expressions in lung tissues were studied.
Conclusions
These findings confirm that the production of the pro- and anti-inflammatory mediators are critically dependent not only upon the different species of bacteria used to establish the experimental infection but also upon the different strains of a specific bacterial species used, i.e., S. pneumoniae in this study. These substantially different host responses were not serotype dependent. Moreover, the profile of lung pro-and anti-inflammatory cytokines within 48 h postinfection, at least in this pneumonia model, was not related to outcome of animals.



Similar content being viewed by others
References
Bartlett JG, Breiman RF, Mandell LA, File TMJ (1998) Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis 26:811–838
Moine P, Vercken JB, Chevret S, Chastang C, Gajdos P, and The French Study Group for Community-Acquired Pneumonia in the Intensive Care Unit (1994) Severe community-acquired pneumonia. Etiology, epidemiology and prognosis factors. Chest 105:1487–1495
Örtqvist A, Hedlund J, Grillner L, Jalonem E, Kallings I, Leinomen M, Kallin M (1990) Aetiology, outcome and prognostic factors in community-acquired pneumonia requiring hospitalization. Eur Respir J 3:1105–1113
Torres A, Serra-Battles J, Ferrer A, Jimenéz P, Celis R, Cobo E, Rodriguez-Roisin R (1991) Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 144:312–318
Johnston RBJ (1991) Pathogenesis of pneumococcal pneumonia. Rev Infect Dis 13 [Suppl 6]:S509–S517
Houldsworth S, Andrew PW, Mitchell TJ (1994) Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect Immun 62:1501–1503
Riesenfeld-Orn I, Wolpe S, Garcia-Bustos JF, Hoffmann MK, Tuomanen E (1989) Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun 57:1890–1893
Simpson SQ, Singh R, Brice DE (1994) Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumor necrosis factor-α production by murine macrophages. Am J Respir Cell Mol Biol 10:284–289
Spellerberg B, Rosenow C, Sha W, Tuomanen E (1996) Pneumococcal cell wall activates NF-κB in human monocytes: aspects distinct from endotoxin. Microb Pathog 20:309–317
Bergeron Y, Ouellet N, Deslauriers A, Simard M, Olivier M, Bergeron MG (1998) Cytokine kinetics and other factors in response to pneumococcal pulmonary infection in mice. Infect Immun 66:912–922
Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K (1997) Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun 65:257–260
Van Der Poll T, Keogh CV, Buurman WA, Lowry SF (1997) Passive immunization against tumor necrosis factor-α impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med 155:603–608
Gordon DL, Hostetter MK (1986) Complement and host defense against microorganisms. Pathology 18:365–375
Gordon DL, Johnson GM, Hostetter MK (1986) Ligand-receptor interactions in the phagocytosis of virulent Streptococcus pneumoniae by polymorphonuclear leukocytes. J Infect Dis 154:619–626
Casadevall A, Pirofski LA (1999) Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun 67:3703–3713
Azoulay-Dupuis E, Rieux V, Muffat-Joly M, Bedos JP, Vallee E, Rivier C, Isturiz R, Carbon C, Moine P (2000) Relationship between capsular type, penicillin susceptibility, and virulence of human Streptococcus pneumoniae isolates in mice. Antimicrob Agents Chemother 44:1575–1577
Moine P, Vallee E, Azoulay-Dupuis E, Bourget P, Bedos JP, Bauchet J, Pocidalo JJ (1994) In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother 38:1953–1958
Amory-Rivier CF, Mohler J, Bedos JP, Azoulay-Dupuis E, Henin D, Muffat-Joly M, Carbon C, Moine P (2000) Nuclear factor-kappaB activation in mouse lung lavage cells in response to Streptococcus pneumoniae pulmonary infection. Crit Care Med 28:3249–3256
Azoulay-Dupuis E, Bedos JP, Vallee E, Pocidalo JJ (1991) Comparative activity of fluorinated quinolones in acute and subacute Streptococcus pneumoniae pneumonia models: efficacy of temafloxacin. J Antimicrob Chemother 28 Suppl C:45–53
Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ (1995) Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol 155:722–729
Laichalk LL, Kunkel SL, Strieter RM, Danforth JM, Bailie MB, Standiford TJ (1996) Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect Immun 64:5211–18
Chernick MR (1999) Bootstrap methods. A practitioner's guide. Willey, New York
Tuomanen E, Rich R, Zak O (1987) Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am Rev Respir Dis 135:869–874
Dehoux MS, Boutten A, Ostinelli J, Seta N, Dombret MC, Crestani B, Deschenes M, Trouillet JL, Aubier M (1994) Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med 150:710–716
Van der Poll T, Keogh CV, Guirao X, et al (1997) Interleukin 6 gene-deficient mice are more susceptible to pneumococcal pneumonia. J Infect Dis 176:439–444
Hessle C, Andersson B, Wold AE (2000) Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun 68:3581–3586
Jiang Y, Magli L, Russo M (1999) Bacterium-dependent induction of cytokines in mononuclear cells and their pathologic consequences in vivo. Infect Immun 67:2125–2130
Silverstein R, Norimatsu M, Morrison DC (1997) Fundamental differences during gram-positive versus gram-negative sepsis become apparent during bacterial challenge of D-galactosamide-treated mice. J Endotoxin Res 4:173–181
Ingalls RR, PA Rice, Qureshi N, Takayama K, Lin JS, Golenbock DT (1995) The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun 63:3125–3130
Jotwani R, Tanaka Y, Watanabe K, Tanaka-Bandoh K, Kato N, Ueno K (1994) Comparison of cytokine induction by lipopolysaccharide of Bacteroides fragilis with Salmonella typhimurium in mice. Microbiol Immunol 38:763–766
Cavaillon JM, Cavaillon NH (1987) Characterization of the induction of human interleukin-1 by endotoxins. In: Brequet P (ed) Lipid mediators in the immunology of burn and sepsis. Plenum, London
Zughaier SM, Ryley HC, Jackson SK (1999) Lipopolysaccharides (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonoas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect Immun 67:1505–1507
Koyama S, Sato E, Nomura H, Kubo K, Miura M, Yamashita T, Nagai S, Izumi T (2000) The potential of various lipopolysaccharides to release IL-8 and G-CSF. Am J Physiol Lung Cell Mol Physiol 278:L658–L666
Engelhard D, Pomeranz S, Gallily R, Strauss N, Tuomanen E (1997) Serotype-related differences in inflammatory response to Streptococcus pneumoniae in experimental meningitis. J Infect Dis 175:979–982
Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, Ferrari L, Simon D (1998) Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun 66:5620–5629
Hava DL, Camilli A (2002) Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol 45:1389–1405
Marchant A, Deviere J, Byl B, de Groote D, Vincent JL, Goldman M (1994) Interleukin-10 production during septicemia. Lancet 343:707–708
Derkx B, Marchant A, Goldman M, Bijlmer R, van Deventer S (1955) High levels of interleukin-10 during the initial phase of fulminant meningococcal septic shock. J Infect Dis 171:229–232
Van Der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF (1996) Iterleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis 174:994–1000
Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gerard C, Delvaux A, De Groote D, Abramowicz D, Velu T, Goldman M (1994) IL-10 controls IFN-g and TNF production during experimental endotoxinemia. Eur J Immunol 24:1167–1171
Florquin S, Amraoui Z, Abramowicz D, Goldman M (1994) Systemic release and protective role of IL-10 in staphylococcal enterotoxin B-induced shock in mice. J Immunol 153:2618–2623
Poll T van der, Marchant A, Buurman WA, Berman L, Keogh CV, Lazarus DD, Nguyen L, Goldman M, Moldawer LL, Lowry SF (1995) Endogenous interleukin 10 protects mice from death during septic peritonitis. J Immunol 155:5397–5401
Poll T van der, Jansen J, Levi M, ten Cate H, ten Cate JW, van Deventer SJH (1994) Regulation of interleukin-10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med 180:1985–1988
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Mohler and P. Moine contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mohler, J., Azoulay-Dupuis, E., Amory-Rivier, C. et al. Streptococcus pneumoniae strain-dependent lung inflammatory responses in a murine model of pneumococcal pneumonia. Intensive Care Med 29, 808–816 (2003). https://doi.org/10.1007/s00134-003-1699-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1699-x