Abstract
Background
This study aimed to evaluate the efficiency of constant dose intravenous administration of tranexamic acid (TXA) in reducing postoperative blood loss, hemoglobin (Hb) concentration, and the number of transfusions in revision hip arthroplasty (RHA).
Methods
The study included 145 consecutive patients who had undergone RHA: a TXA group (75 patients) who received two doses of TXA (1.0 g 15 min before skin incision and 1.0 g during wound closure) and a no-TXA group (70 patients). Percentage blood loss and quantitative blood loss were calculated.
Results
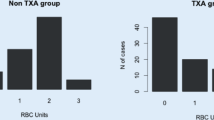
The percentage blood loss (23.82 ± 10.6% vs. 39.17 ± 15.1%; P < 0.001), Hb drop (2.9 ± 1.14 g/dL vs. 4.22 ± 1.4 g/dL; P < 0.001), and total blood loss (1030 ± 477 mL vs. 1736 ± 761 mL; P < 0.001) were significantly lower in the TXA group than in the no-TXA group on postoperative day 1. Percentage blood loss (37.5 ± 10.4% vs. 43.1 ± 12.5%; P < 0.01), Hb drop (4.64 ± 1.5 g/dL vs. 5.22 ± 1.6 g/dL; P < 0.01) and total blood loss (1639 ± 543 mL vs. 1908 ± 681 mL; P = 0.02) were significantly lower in the TXA group than in the no-TXA group on the 5th postoperative day. The blood transfusion requirements were lower in the TXA group than those in the no-TXA group (30.7% vs. 71.4% of patients; P < 0.001), with a lower transfusion per patient ratio of 0.55 in the TXA group and 1.4 in the no-TXA group. No postoperative complications were associated with TXA administration, including deep-vein thrombosis and pulmonary embolism.
Conclusion
Administration of TXA is an effective method to reduce perioperative blood loss, Hb drop and the number of transfusions in RHA.
Zusammenfassung
Hintergrund
Ziel dieser Studie war es, die Wirksamkeit der intravenösen Gabe von Tranexamsäure (TXA) in konstanter Dosis bei der Reduzierung des postoperativen Blutverlusts, der Hämoglobinkonzentration (Hb) und der Anzahl der Transfusionen bei der Revisions-Hüftendoprothetik (RHA) zu untersuchen.
Methoden
Die Studie schloss 145 konsekutive Patienten ein, die sich einer RHA unterzogen hatten: eine TXA-Gruppe (75 Patienten), die 2 Dosen TXA erhielt (1,0 g 15 min vor der Hautinzision und 1,0 g während des Wundverschlusses) und eine Gruppe ohne TXA (70 Patienten). Der prozentuale Blutverlust und der quantitative Blutverlust wurden mathematisch berechnet.
Ergebnisse
Der durchschnittliche Blutverlust (23,82 ± 10,6 % vs. 39,17 ± 15,1 %; p < 0,001), der Hb-Abfall (2,9 ± 1,14 g/dl vs. 4,22 ± 1,4 g/dl; p < 0,001) und der Gesamtblutverlust (1030 ± 477 ml vs. 1736 ± 761 ml; p < 0,001) waren in der TXA-Gruppe am postoperativen Tag 1 signifikant niedriger als in der Gruppe ohne TXA. Am 5. postoperativen Tag waren der prozentuale Blutverlust (37,5 ± 10,4 % vs. 43,1 ± 12,5 %; p < 0,01), der Hb-Abfall (4,64 ± 1,5 g/dl vs. 5,22 ± 1,6 g/dl; p < 0,01) und der Gesamtblutverlust (1639 ± 543 ml vs. 1908 ± 681 ml; p = 0,02) in der TXA-Gruppe signifikant niedriger als in der Gruppe ohne TXA. Der Bluttransfusionsbedarf war in der TXA-Gruppe niedriger als in der Gruppe ohne TXA (30,7 % vs. 71,4 % der Patienten; p < 0,001), mit einem niedrigeren Verhältnis von Transfusionen pro Patient von 0,55 in der TXA-Gruppe und 1,4 in der Gruppe ohne TXA. Bei der Verabreichung von TXA traten keine postoperativen Komplikationen auf, einschließlich tiefer Venenthrombose und Lungenembolie.
Schlussfolgerung
Die Verabreichung von TXA ist eine wirksame Methode, um den perioperativen Blutverlust, den Hb-Abfall und die Anzahl der Transfusionen bei der RHA zu reduzieren.
Similar content being viewed by others
Abbreviations
- AABB:
-
American Association of Blood Banks
- DVT:
-
Deep vein thrombosis
- HCT:
-
Hematocrit
- Hb:
-
Hemoglobin
- PE:
-
Pulmonary embolism
- RBC:
-
Red blood cells
- RHA:
-
Revision hip arthroplasty
- THA:
-
Total hip arthroplasty
- TXA:
-
Tranexamic acid
References
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785
Bridgens JP, Evans CR, Dobson PM, Hamer AJ (2007) Intraoperative red blood-cell salvage in revision hip surgery. A case-matched study. J Bone Joint Surg Am 89:270–275
Callaghan JJ, O’Rourke MR, Liu SS (2005) Blood management: Issues and options. J Arthroplasty 20:51–54
Harris RN, Moskal JT, Capps SG (2015) Does tranexamic acid reduce blood transfusion cost for primary total hip arthroplasty? A case-control study. J Arthroplasty 30:192–195
Holt JB, Miller BJ, Callaghan JJ, Clark CR, Willenborg MD, Noiseux NO et al (2016) Minimizing blood transfusion in total hip and knee arthroplasty through a multimodal approach. J Arthroplasty 31:378–382
Lin ZX, Woolf SK (2016) Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics 39:119–130
Sano M, Hakusui H, Kojima C, Akimoto T (1976) Absorption and excretion of tranexamic acid following intravenous, intramuscular and oral administrations in healthy volunteers. Jpn J Clin Pharmacol Ther 7:375–382
Calapai G, Gangemi S, Mannucci C, Miniullo PL, Casciaro M et al (2015) Systematic review of tranexamic acid adverse reactions. J Pharmacovigilance 3:171
Fígar A, Mc Loughlin S, Slullitel PA et al (2017) Influence of single-dose intravenous tranexamic acid on total hip replacement. Orthopade 46:359–365
Zhang Y, Zhang L, Ma X et al (2016) What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Orthopade 45:616–621
Xie J, Hu Q, Huang Q, Ma J, Lei Y, Pei F et al (2017) Comparison of intravenous versus topical tranexamic acid in primary total hip and knee arthroplasty: an updated meta-analysis. Thromb Res 153:28–36
Hines JT, Hernandez NM, Amundson AW et al (2019) Intravenous tranexamic acid safely and effectively reduces transfusion rates in revision total hip arthroplasty. Bone Joint J 101-B(6_Supple_B):104–109
Fillingham YA, Darrith B, Calkins TE et al (2019) Group. 2019 Mark Coventry Award: a multicentre randomized clinical trial of tranexamic acid in revision total knee arthroplasty: does the dosing regimen matter? Bone Joint J 101-B(7_Supple_C):10–16
Koo BN, Kwon MA, Kim SH et al (2019) Korean clinical practice guideline for perioperative red blood cell transfusion from Korean Society of Anesthesiologists. Korean J Anesthesiol 72(2):91–118
Gross JB (1983) Estimating allowable blood loss: Corrected for dilution. Anesthesiology 58:277–280
Mercuriali F, Inghilleri G (1996) Proposal of an algorithm to help the choice of the best transfusion strategy. Curr Med Res Opin 13:465–478
Nadler SB, Hidalgo JH, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224–232
Hourlier H, Fennema P (2014) Single tranexamic acid dose to reduce perioperative morbidity in primary total hip replacement: a randomised clinical trial. Hip Int 24:63–68
Zeng Y, Si HB, Shen B, Yang J, Zhou ZK, Kang PD et al (2017) Intravenous combined with topical administration of tranexamic acid in primary total hip arthroplasty: A randomized controlled trial. Orthop Surg 9:174–179
Wang C, Kang P, Ma J, Yue C, Xie J, Pei F et al (2016) Single-dose tranexamic acid for reducing bleeding and transfusions in total hip arthroplasty: A double-blind, randomized controlled trial of different doses. Thromb Res 141:119–123
Park KJ, Couch CG, Edwards PK, Siegel ER, Mears SC, Barnes CL et al (2016) Tranexamic acid reduces blood transfusions in revision total hip arthroplasty. J Arthroplasty 31:2850–2855e1
Kazi HA, Fountain JR, Thomas TG, Carroll FA (2012) The effect of bolus administration of tranexamic acid in revision hip arthroplasty. Hip Int 22:615–620
Wu YG, Zeng Y, Yang TM, Si HB, Cao F, Shen B et al (2016) The efficacy and safety of combination of intravenous and topical tranexamic acid in revision hip arthroplasty: a randomized, controlled trial. J Arthroplasty 31:2548–2553
Phillips SJ, Chavan R, Porter ML, Kay PR, Hodgkinson JP, Purbach B et al (2006) Does salvage and tranexamic acid reduce the need for blood transfusion in revision hip surgery? J Bone Joint Surg Br 88:1141–1142
Reichel F, Peter C, Ewerbeck V et al (2018) Reducing blood loss in revision total hip and knee arthroplasty: tranexamic acid is effective in aseptic revisions and in second-stage reimplantations for Periprosthetic infection. Biomed Res Int. https://doi.org/10.1155/2018/3891870
Sukeik M, Alshryda S, Powell J et al (2020) The effect of tranexamic acid on wound complications in primary total hip arthroplasty: a meta-analysis. Surgeon 18(1):53–61
Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM et al (2015) Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty 30:272–276
Author information
Authors and Affiliations
Contributions
DG, PD, TO, JK designed the research, developed the concept of the article, acquired the data and critically revised it for important intellectual content. DG, SM, BK analyzed the data. DG, DM, JK contributed to interpretation of data. DG, SM prepared and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
D. Grzelecki, P. Dudek, T. Okoń, D. Marczak, B. Kordasiewicz, M. Sibiński and J. Kowalczewski declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Grzelecki, D., Dudek, P., Okoń, T. et al. Efficacy of intravenous tranexamic acid administration in revision hip arthroplasty. Orthopäde 50, 464–470 (2021). https://doi.org/10.1007/s00132-020-03959-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00132-020-03959-9