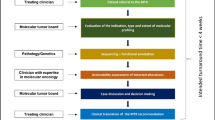
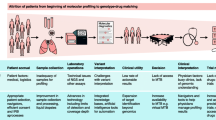
Abstract
Efforts to translate information from sequencing-based tumor genetic characterization into treatment personalization are becoming widespread in the clinic. However, for them to be successful and consequently lead to improved treatment outcomes, familiarity with predictive biomarkers and the associated treatment landscape is mandatory. The purpose of this review is to provide a structured overview of established biomarkers and associated genetic alterations ranging from single-gene to complex biomarkers, as well as to summarize the presently available clinical evidence on their predictive value with respect to currently approved targeted therapies in advanced breast and gynecologic malignancies. Importantly, apart from predicting response, sequencing-derived biomarkers can also inform on the lack of benefit from or even resistance to certain targeted therapies. In addition to providing an overview on how tumor genetics can guide selection of targeted therapies within their approved indication, this review also provides biomarker examples and related evidence which could suggest their potential off-label use, particularly in situations where tumor sequencing reveals a potentially actionable alteration but no other satisfactory treatment options remain. Moreover, another practical consequence of tumor sequencing for clinicians is that it can also facilitate and expedite access of patients to innovative treatment options within clinical trials, as it can be used as a powerful tool for patient stratification, especially for genomically driven and biomarker-stratified trials. In conclusion, all of the above illustrate the transformative potential and impact that tumor molecular profiling is having on the clinical management of advanced breast and gynecologic malignancies.
Zusammenfassung
Die genetische Charakterisierung von Tumoren durch Sequencing nimmt zunehmend Eingang in die Klinik als personalisierte Medizin. Um aber diese Techniken erfolgreich für die Behandlung einsetzen zu können, sind gute Kenntnisse über prädiktive Biomarker und die daraus ableitbaren Behandlungsstrategien unumgänglich. In der vorliegenden Arbeit werden deshalb die Zusammenhänge zwischen genetischer Tumorcharakterisierung und etablierten prädiktiven Biomarkern dargestellt. Außerdem wird auf ihre prädiktive Bedeutung für bereits klinisch verwendete Targettherapien insbesondere bei fortgeschrittenen Mammakarzinomen und anderen gynäkologischen Malignomen eingegangen. Darüber hinaus können genetische Signaturen nicht nur als prädiktive Biomarker eingesetzt werden, sondern auch helfen, die Wirksamkeit neuer Therapien zu bewerten. Auch können sich aus dieser genetischen Diagnostik neue Behandlungsmöglichkeiten mit Medikamenten im „off-label use“ ergeben, wenn keine anderen etablierten Behandlungsverfahren mehr eingesetzt werden können. Eine genetische Tumorcharakterisierung kann Patienten auch den Zugang zu neuen Therapiestudien eröffnen, da die Tumorgenetik neue Wege der Stratifizierung insbesondere in Studien, die gezielt nach genetischen Befunden stratifizieren, ermöglicht. So bietet eine molekulargenetische Charakterisierung von Tumoren zahlreiche neue Ansätze für die Onkologie weg von einer ausschließlich organbezogenen Betrachtungsweise hin zu einer Tumorklassifizierung basierend auf genetischen Mustern. Damit wird das transformative Potenzial und der Einfluss des molekularen Profilings von Tumoren in Bezug auf die klinische Versorgung fortgeschrittener Mammakarzinome und anderer gynäkologischer Malignome veranschaulicht.
Similar content being viewed by others
References
Weinstein JN, Collisson EA, Mills GB et al (2013) The cancer genome atlas pan-cancer analysis project. Nat Genet 45:1113–1120
ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium (2020) Pan-cancer analysis of whole genomes. Nature 578:82–93
Horak P, Klink B, Heining C et al (2017) Precision oncology based on omics data: the NCT Heidelberg experience. Int J Cancer 141:877–886
Kaufman B, Shapira-Frommer R, Schmutzler RK et al (2015) Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33:244–250
Lightfoot M, Montemorano L, Bixel K (2020) PARP inhibitors in gynecologic cancers: what is the next big development? Curr Oncol Rep 22:29
Robson M, Im SA, Senkus E et al (2017) Olaparib for metastatic breast cancer in patients with a Germline BRCA mutation. N Engl J Med 377:523–533
Litton JK, Rugo HS, Ettl J et al (2018) Talazoparib in patients with advanced breast cancer and a Germline BRCA mutation. N Engl J Med 379:753–763
González-Martín A, Pothuri B, Vergote I et al (2019) Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391–2402
Coleman RL, Oza AM, Lorusso D et al (2017) Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:1949–1961
Tutt ANJ, Garber JE, Kaufman B et al (2021) Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 384:2394–2405
Swisher EM, Kwan TT, Oza AM et al (2021) Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat Commun 12:2487
Tung NM, Robson ME, Ventz S et al (2020) TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38:4274–4282
Bertucci F, Ng CKY, Patsouris A et al (2019) Genomic characterization of metastatic breast cancers. Nature 569:560–564
André F, Ciruelos EM, Juric D et al (2021) Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR‑1. Ann Oncol 32:208–217
Rugo HS, Lerebours F, Ciruelos E et al (2021) Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol 22:489–498
Rugo HS, Neven P, Saffie I et al (2021) Alpelisib + fulvestrant in patients with PIK3CA-mutated, HR+, HER2- advanced breast cancer (ABC) who received chemotherapy or endocrine therapy (ET) as immediate prior treatment: BYLieve Cohort C primary results and exploratory biomarker analyses. SABCS 2021 Abstract #PD13-05
Sharma P, Abramson VG, O’dea A et al (2021) Clinical and biomarker results from phase I/II study of PI3K inhibitor alpelisib plus nab-paclitaxel in HER2-negative metastatic breast cancer. Clin Cancer Res 27:3896–3904
Kandoth C, Schultz N, Cherniack AD et al (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497:67–73
Ojesina AI, Lichtenstein L, Freeman SS et al (2014) Landscape of genomic alterations in cervical carcinomas. Nature 506:371–375
Rinne N, Christie EL, Ardasheva A et al (2021) Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. CDR 4:573–595
Juric D, Rodon J, Tabernero J et al (2018) Phosphatidylinositol 3‑Kinase α‑selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J Clin Oncol 36:1291–1299
Hortobagyi GN, Chen D, Piccart M et al (2016) Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2‑negative advanced breast cancer: results from BOLERO‑2. J Clin Oncol 34:419–426
André F, Hurvitz S, Fasolo A et al (2016) Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2‑overexpressing metastatic breast cancers: combined exploratory Biomarker analysis from BOLERO‑1 and BOLERO‑3. J Clin Oncol 34:2115–2124
Basho RK, Gilcrease M, Murthy RK et al (2017) Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: evidence from a phase 1 trial of mTOR inhibition in combination with Liposomal Doxorubicin and Bevacizumab. JAMA Oncol 3:509–515
Hyman DM, Piha-Paul SA, Won H et al (2018) HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189–194
Oaknin A, Friedman CF, Roman LD et al (2020) Neratinib in patients with HER2-mutant, metastatic cervical cancer: findings from the phase 2 SUMMIT basket trial. Gynecol Oncol 159:150–156
Jhaveri K, Park H, Waisman J et al (2021) Neratinib + fulvestrant + trastuzumab for hormone receptor-positive, HER2-mutant metastatic breast cancer and neratinib + trastuzumab for triple-negative disease: latest updates from the SUMMIT trial. SABCS 2021 Abstract #GS4-10
Westphalen CB, Krebs MG, Le Tourneau C et al (2021) Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis Oncol 5:69
Doebele RC, Drilon A, Paz-Ares L et al (2020) Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 21:271–282
Hong DS, Dubois SG, Kummar S et al (2020) Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21:531–540
Rosen EY, Italiano A, Juric D et al (2020) Efficacy and safety of larotrectinib in patients with TRK fusion breast cancer. SABCS 2020 Abstract #PS11-06
Yarchoan M, Hopkins A, Jaffee EM (2017) Tumor mutational burden and response rate to PD‑1 inhibition. N Engl J Med 377:2500–2501
Marabelle A, Fakih M, Lopez J et al (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21:1353–1365
Klempner SJ, Fabrizio D, Bane S et al (2020) Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. The Oncol 25:e147–e159
Mcgrail DJ, Pilié PG, Rashid NU et al (2021) High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol 32:661–672
Rousseau B, Foote MB, Maron SB et al (2021) The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med 384:1168–1170
Barroso-Sousa R, Keenan TE, Pernas S et al (2020) Tumor mutational burden and PTEN alterations as molecular correlates of response to PD-1/L1 blockade in metastatic triple-negative breast cancer. Clin Cancer Res 26:2565–2572
Friedman CF, Hainsworth JD, Kurzrock R et al (2021) Atezolizumab treatment of tumors with high tumor mutational burden from mypathway, a multicenter, open-label, phase IIa multiple basket study. Cancer Discov 12:654–669. https://doi.org/10.1158/2159-8290.CD-21-0450
Le DT, Durham JN, Smith KN et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD‑1 blockade. Science 357:409–413
Marabelle A, Le DT, Ascierto PA et al (2020) Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1–10
O’malley DM, Bariani GM, Cassier PA et al (2022) Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol 40:752–761
Pellegrino B, Mateo J, Serra V et al (2019) Controversies in oncology: are genomic tests quantifying homologous recombination repair deficiency (HRD) useful for treatment decision making? ESMO Open 4:e480
Telli ML, Timms KM, Reid J et al (2016) Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res 22:3764–3773
Ray-Coquard I, Pautier P, Pignata S et al (2019) Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416–2428
Tutt A, Tovey H, Cheang MCU et al (2018) Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 24:628–637
Lin KK, Harrell MI, Oza AM et al (2019) BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov 9:210–219
Waks AG, Cohen O, Kochupurakkal B et al (2020) Reversion and non-reversion mechanisms of resistance to PARP inhibitor or platinum chemotherapy in BRCA1/2-mutant metastatic breast cancer. Ann Oncol 31:590–598
Weigelt B, Comino-Méndez I, De Bruijn I et al (2017) Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin Cancer Res 23:6708–6720
Tobalina L, Armenia J, Irving E et al (2021) A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol 32:103–112
Pettitt SJ, Frankum JR, Punta M et al (2020) Clinical BRCA1/2 reversion analysis identifies hotspot mutations and predicted neoantigens associated with therapy resistance. Cancer Discov 10:1475–1488
Brett JO, Spring LM, Bardia A et al (2021) ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 23:85
Fribbens C, O’leary B, Kilburn L et al (2016) Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 34:2961–2968
Gaillard SL, Andreano KJ, Gay LM et al (2019) Constitutively active ESR1 mutations in gynecologic malignancies and clinical response to estrogen-receptor directed therapies. Gynecol Oncol 154:199–206
Spoerke JM, Gendreau S, Walter K et al (2016) Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 7:11579
Bidard FC, Hardy-Bessard AC, Bachelot T et al (2021) Fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- metastatic breast cancer patients: results of PADA‑1, a UCBG-GINECO randomized phase 3 trial. SABCS 2021 Abstract #GS3-05
Chandarlapaty S, Chen D, He W et al (2016) Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO‑2 clinical trial. JAMA Oncol 2:1310–1315
Li Z, Razavi P, Li Q et al (2018) Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cells 34:893–905.e898
Formisano L, Lu Y, Servetto A et al (2019) Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun 10:1373
Drago JZ, Formisano L, Juric D et al (2019) FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR(+)) breast cancer. Clin Cancer Res 25:6443–6451
Velimirovic M, Gerratana L, Davis AA et al (2021) Genomic predictors of rapid progression to first line endocrine and CDK4/6 inhibitor combination therapy in patients with estrogen receptor positive (ER+) HER‑2 negative (HER2-) advanced breast cancer (ABC). SABCS 2021 Abstract #P2-07-02
Wander SA, Han HS, Zangardi ML et al (2021) Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: a multicenter experience. J Natl Compr Canc Netw. https://doi.org/10.6004/jnccn.2020.7662
Brett JO, Dubash TD, Niemierko A et al (2021) Association between co-existing genomic alterations and abemaciclib benefit in patients with metastatic hormone receptor-positive breast cancer with ESR1 mutations following disease progression on prior endocrine therapy plus palbociclib or ribociclib. SABCS 2021 Abstract #PD2-03
Hlevnjak M, Schulze M, Elgaafary S et al (2021) CATCH: a prospective precision oncology trial in metastatic breast cancer. JCO Precis Oncol. https://doi.org/10.1200/PO.20.00248
Horak P, Heining C, Kreutzfeldt S et al (2021) Comprehensive genomic and transcriptomic analysis for guiding therapeutic decisions in patients with rare cancers. Cancer Discov 11:2780–2795
Miao L, Zhang Y, Huang L (2021) mRNA vaccine for cancer immunotherapy. Mol Cancer 20:41
Sahin U, Derhovanessian E, Miller M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547:222–226
Magbanua MJM, Swigart LB, Wu HT et al (2021) Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol 32:229–239
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Hlevnjak declares receiving speaker’s fee from Merck Sharp & Dohme (MSD).
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Redaktion
Thomas Strowitzki, Heidelberg
Klaus Diedrich, Lübeck

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Hlevnjak, M. Tumor genetics and individualized therapy. Gynäkologie 55, 424–431 (2022). https://doi.org/10.1007/s00129-022-04931-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00129-022-04931-8