Abstract
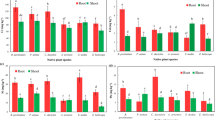
The present study aims to signify the role of Pyxine cocoes (Sw.) Nyl. (P. cocoes) as cadmium (Cd) biomonitor in atmosphere. This was achieved by quantifying the amount of Cd accumulated in transplanted P. cocoes, when stimulated with known concentrations of Cd (5µM, 50µM, 100µM, 150µM and 200µM) at increasing intervals of time up-to 40 days. All the five concentrations exhibited increasing trend of accumulation with time. As depicted by Pearson’s Correlation (at p < 0.001), anti-oxidative enzymes (superoxide dismutase r= -0.812, ascorbate peroxidase r= -0.802, catalase r= -0.757) and electrical conductivity (r = 0.693) were the most efficient parameters to depict increased Cd presence in atmosphere. In the current study, accumulation of Cd by transplanted lichen has been first time analyzed by biosorption kinetics. The uptake of Cd by P. cocoes followed pseudo-second-order kinetics (range of R22 value was 0.969–0.998). The marker parameters in combination with the ability to accrue Cd fortifies P. cocoes’s role as a biomonitor.


Similar content being viewed by others
References
ATSDR (Agency for Toxic Substances and Disease Registry) (2012). Toxicological profile for cadmium.
Arnon DI (1949) Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiology 24:1–15. https://doi.org/10.1104/pp.24.1.1
Bačkor M, Gibalová A, Bud’ová J, Mikeš J, Solár P (2006) Cadmium-induced stimulation of stress-protein hsp70 in lichen photobiont Trebouxia erici. Plant Growth Regulation 50:159–164. https://doi.org/10.1007/s10725-006-9112-8
Bačkor M, Pawlik-Skowrońska B, Bud’ová J, Skowroński T (2007a) Response to copper and cadmium stress in wild-type and copper tolerant strains of the lichen alga Trebouxia erici: Metal accumulation, toxicity and non-protein thiols. Plant Growth Regulation 52:17–27. https://doi.org/10.1007/s10725-007-9173-3
Bačkor M, Váczi P, Barták M, Buďová J, Dzubaj A (2007b) Uptake, photosynthetic characteristics and membrane lipid peroxidation levels in the lichen photobiont Trebouxia erici exposed to copper and cadmium. Bryologist 110:100–107. https://doi.org/10.1639/0007-2745(2007)110[100:UPCAML]2.0.CO;2
Bačkor M, Kováčik J, Piovár J, Pisani T, Loppi S (2010) Physiological aspects of cadmium and nickel toxicity in the lichens peltigera rufescens and cladina arbuscula subsp. mitis. Water, Air, and Soil Pollution 207:253–262. https://doi.org/10.1007/s11270-009-0133-6
Bajpai R, Pandey AK, Deeba F, Upreti DK, Nayaka S, Pandey V (2012) Physiological effects of arsenate on transplant thalli of the lichen Pyxine cocoes (Sw.) Nyl. Environmental Science and Pollution Research 19:1494–1502. https://doi.org/10.1007/s11356-011-0628-8
Bajpai R, Shukla V, Singh N, Rana TS, Upreti DK (2015) Physiological and genetic effects of chromium (+ VI) on toxitolerant lichen species, Pyxine cocoes. Environmental Science and Pollution Research 22:3727–3738. https://doi.org/10.1007/s11356-014-3622-0
Beckett RP, Brown DH (1984) The control of cadmium uptake in the lichen genus Peltigera. Journal of Experimental Botany 35(7):1071–1082.
Bernanrd A (2008) Cadmium & its adverse effects on human health. Indian Journal of Medical Research 128(4):557–564.
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Analytical Biochemistry 161:559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiology 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Calcott MJ, Ackerley DF, Knight A, Keyzers RA, Owen JG (2018) Secondary metabolism in the lichen symbiosis. Chemical Society Reviews 47(5):1730–1760. https://doi.org/10.1039/c7cs00431a
Cardinaels C, Put C, Vanassche F, Clijsters H (1984) The superoxide-dismutase as a biochemical indicator, discriminating between zinc and cadmium toxicity. Archives Internationales de Physiologie de Biochimie et de Biophysique 92(5):PF2–PF3.
Dat J, Vandenabeele S, Vranova E, Montaqu DV, Inz MF, Breusegen V (2000) Dual action of the active oxygen species during plant stress responses. Cellular and Molecular Life Sciences 57:779–795.
Di Toppi LS, Marabottini R, Vattuone Z, Musetti R, Favali MA, Sorgonà A, Badiani M (2005) Cell wall immobilisation and antioxidant status of Xanthoria parietina thalli exposed to cadmium. Functional Plant Biology 32:611–618. https://doi.org/10.1071/FP04237
Garty J (2001) Biomonitoring atmospheric heavy metals with lichens: Theory and application. Critical Reviews in Plant Sciences 20:309–371. https://doi.org/10.1080/20013591099254
Gonzalez CM, Pignata ML (1994) The influence of air pollution on soluble proteins, chlorophyll degradation, mda, sulphur and heavy metals in a transplanted lichen. Chemistry and Ecology 9:105–113. https://doi.org/10.1080/02757549408038568
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochemistry 34:451–465.
IARC (2012) IARC monographs on the evaluation of carcinogenic risks to humans, volume 100c: Cadmium and cadmium compounds. IARC Monographs 1993:121–145.
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284(5414):654–657.
Koyuncu H, Kul AR (2020) Removal of methylene blue dye from aqueous solution by nonliving lichen (Pseudevernia furfuracea (L.) Zopf.), as a novel biosorbent. Applied Water Science 10(2):1–14.
Kranner I, Zorn M, Turk B, Wornik S, Beckett RP, Batič F (2003) Biochemical traits of lichens differing in relative desiccation tolerance. New Phytologist 160(1):167–176. https://doi.org/10.1046/j.1469-8137.2003.00852.x
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens. Handlingar 24:1–39.
Loppi S, Di Lucia A, Vannini A, Ancora S, Monaci F, Paoli L (2020) Uptake and release of copper ions in epiphytic lichens. Biologia 75:1547–1552. https://doi.org/10.2478/s11756-020-00522-x
López Berdonces MA, Higueras PL, Fernández-Pascual M, Borreguero AM, Carmona M (2016) The role of native lichens in the biomonitoring of gaseous mercury at contaminated sites. Journal of Environmental Management 186:207–213. https://doi.org/10.1016/j.jenvman.2016.04.047
Lowry, Randall RJ, Lewis A (1951) Méthode de Lowry. Journal of Biological Chemistry 193:265–275
Marques AP, Freitas MC, Wolterbeek HT, Steinebach OM, Verburg T, De Goeij JJ (2005) Cell-membrane damage and element leaching in transplanted Parmelia sulcata lichen related to ambient SO2, temperature, and precipitation. Environmental Science and Technology 39:2624–2630. https://doi.org/10.1021/es0498888
Mondal NK, Kundu M (2016) Biosorption of Fluoride from Aqueous Solution Using Lichen and Its Ca-Pretreated Biomass. Water Conservation Science Enggineering 1:143–160. https://doi.org/10.1007/s41101-016-0009-8
Monnet F, Bordas F, Deluchat V, Baudu M (2006) Toxicity of copper excess on the lichen Dermatocarpon luridum: Antioxidant enzyme activities. Chemosphere 65:1806–1813. https://doi.org/10.1016/j.chemosphere.2006.04.022
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nylander MW (1866). Les lichens du Jardin du Luxembourg. Bulletin de la Société botanique de France 13(7):364–371.
Orlowski C, Piotrowski JK (2003) Biological levels of cadmium and zinc in the small intestine of non-occupationally exposed human subjects. Human and Experimental Toxicology 22:57–63.https://doi.org/10.1191/0960327103ht326oa
Osyczka P, Rola K (2019) Integrity of lichen cell membranes as an indicator of heavy-metal pollution levels in soil. Ecotoxicology and Environmental Safety 174:26–34. https://doi.org/10.1016/j.ecoenv.2019.02.054
Parsons TR, Maita Y, Lalli CMA (1984) Manual of chemical and biological methods for seawater analysis. Oxford, Pergamon
Prasad TK, Anderson MD, Martin BA, Steward CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. The Plant Cell 6(1):65–74.
Reis MA, Alves LC, Freitas MC, VanOs B, Wolterbeek HT (1999) Lichens (Parmelia sulcata) time response model to environmental availability. Science of the Total Environment 232:105–115.
Sanità Di Toppi L, Musetti R, Vattuone Z, Pawlik-Skowrońska B, Fossati F, Bertoli L, Badiani M, Favali MA (2005) Cadmium distribution and effects on ultrastructure and chlorophyll status in photobionts and mycobionts of Xanthoria parietina. Microscopy Research and Technique 66:229–238. https://doi.org/10.1002/jemt.20166
Sanità di Toppi L, Pawlik-Skowrońska B, Vurro E (2008) First and second line mechanisms of cadmium detoxification in the lichen photobiont Trebouxia impressa (Chlorophyta). Environmental Pollution 151:280–286. https://doi.org/10.1016/j.envpol.2007.06.010
Sari A, Tuzen M, Uluözlü ÖD, Soylak M (2007) Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochemical Engineering Journal 37(2):151–158. https://doi.org/10.1016/j.bej.2007.04.007
Seminara A, Fritz J, Brenner MP, Pringle A (2018) A universal growth limit for circular lichens. Journal of The Royal Society Interface 15(143).
Tay T, Candan M, Erdem M, Çimen Y, Türk H (2009) Biosorption of cadmium ions from aqueous solution onto non-living Lichen Ramalina fraxinea biomass. CLEAN–Soil, Air, Water 37(3):249–255.
Uluozlu OD, Sari A, Tuzon M (2010) Biosorption of antimony from aqueous solution by lichen (Physcia tribacia) biomass. Chemical Engineering Journal 163:382–388. https://doi.org/10.1016/j.cej.2010.08.022
World Health Organization (2010) Exposure to cadmium: a major public health concern. Preventing Disease Through Healthy Environments 3–6.
Yemets O, Gauslaa Y, Solhaug KA (2015) Monitoring with lichens - Conductivity methods assess salt and heavy metal damage more efficiently than chlorophyll fluorescence. Ecological Indicators 55:59–64. https://doi.org/10.1016/j.ecolind.2015.03.015
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50.
Acknowledgements
The authors are thankful to the Directors of Institute of Engineering and Technology, Lucknow, and CSIR-National Botanical Research Institute, Lucknow, India for providing laboratory facilities to carry out this research work. We are also thankful to the farmers for allowing us to collect samples from their Mango orchard situated near Tokhaiedih, Bahraich, India.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, B.K., Shukla, A.K., Upreti, D.K. et al. Accumulation of Cadmium in Transplanted Lichen Pyxine cocoes (Sw.) Nyl., with Reference to Physiochemical Variation and Kinetics of Cadmium Biosorption. Bull Environ Contam Toxicol 110, 67 (2023). https://doi.org/10.1007/s00128-023-03710-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03710-y