Abstract
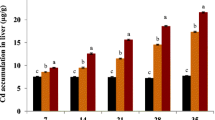
The accumulation, elimination and effect of heavy metals on plasma cortisol levels in Oreochromis sp. were studied in the exposure and recovery phases. In the exposure phase, the mean rate of accumulation in the tissues was in the order gill > liver > muscle for Pb exposure and muscle > liver > gill for As exposure. In the recovery phase, the order of elimination in the tissues was gill > liver > muscle for Pb and liver > gill > muscle for As. The amount of cortisol secreted by the Oreochromis sp. after Pb or As treatment was lower compared to Oreochromis sp. in the control group during the exposure phase. In the recovery phase, plasma cortisol levels in Oreochromis sp. increased in all Pb treatment groups while it continuously decreased in all As treatment groups. A fish affected by As has obvious difficulty recovering from the stress response. It was concluded that exposure to the tested concentrations of Pb and As over 20 days could be a potent endocrine disruptor, which may lead to adverse impacts on the health of the Oreochromis sp.



Similar content being viewed by others
References
Agah H, Leermakers M, Elskens M, Fatemi SMR, Baeyens W (2008) Accumulation of trace metals in the muscle and liver tissues of five fish species from the Persian Gulf. Environ Monit Assess 157:499. doi:10.1007/s10661-008-0551-8
Altındağ A, Yiğit S (2005) Assessment of heavy metal concentrations in the food web of lake Beyşehir, Turkey. Chemosphere 60:552–556. doi:10.1016/j.chemosphere.2005.01.009
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202. doi:10.1016/j.aquatox.2005.08.015
Bears H, Richards JG, Schulte PM (2006) Arsenic exposure alters hepatic arsenic species composition and stress-mediated gene expression in the common killifish (Fundulus heteroclitus). Aquat Toxicol 77:257–266. doi:10.1016/j.aquatox.2005.12.008
Brodeur JC, Sherwood G, Rasmussen JB, Hontela A (1997) Impaired cortisol secretion in yellow perch (Perca flavescens) from lakes contaminated by heavy metals: in vivo and in vitro assessment. Can J Fish Aquat Sci 54:2752–2758. doi:10.1139/f97-200
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121:129–136. doi:10.1016/S0269-7491(02)00194-X
Claiborne JB, Edwards SL, Morrison-Shetlar AI (2002) Acid–base regulation in fishes: cellular and molecular mechanisms. J Exp Zool 293:302–319. doi:10.1002/jez.10125
Çoğun HY, Şahin M (2013) The effect of Lead and Zeolite on hematological and some biochemical parameters in Nile Fish (Oreochromis niloticus). In: Silva-Opps M (ed) Current progress in biological research. InTech, Rijeka. doi:10.5772/53076
Ebrahimpour M, Mushrifah I (2010) Seasonal variation of Cadmium, Copper, and Lead Concentrations in Fish from a freshwater lake. Biol Trace Elem Res 138:190–201. doi:10.1007/s12011-009-8596-2
El-Moselhy KM, Othman AI, Abd El-Azem H, El-Metwally MEA (2014) Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt J Basic Appl Sci 1:97–105. doi:10.1016/j.ejbas.2014.06.001
Evans DW, Dodoo DK, Hanson PJ (1993) Trace element concentrations in fish livers: implications of variations with fish size in pollution monitoring. Mar Pollut Bull 26:329–334. doi:10.1016/0025-326X(93)90576-6
Fowler SW, Readman JW, Oregioni B, Villeneuve JP, McKay K (1993) Petroleum hydrocarbons and trace metals in nearshore Gulf sediments and biota before and after the 1991 war: an assessment of temporal and spatial trends. Mar Pollut Bull 27:171–182. doi:10.1016/0025-326X(93)90022-C
Gregory TR, Wood CM (1999) The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and Swimming performance of juvenile rainbow trout. Physiol Biochem Zool 72:286–295. doi:10.1086/316673
Has-Schön E, Bogut I, Strelec I (2006) Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch Environ Contam Toxicol 50:545–551. doi:10.1007/s00244-005-0047-2
Has-Schön E, Bogut I, Rajković V, Bogut S, Čačić M, Horvatić J (2008) Heavy metal distribution in tissues of six fish species included in human diet, inhabiting freshwaters of the nature park “Hutovo Blato” (Bosnia and Herzegovina). Arch Environ Contam Toxicol 54:75–83. doi:10.1007/s00244-007-9008-2
Has-Schön E, Bogut I, Vuković R, Galović D, Bogut A, Horvatić J (2015) Distribution and age-related bioaccumulation of lead (Pb), mercury (Hg), cadmium (Cd), and arsenic (As) in tissues of common carp (Cyprinus carpio) and European catfish (Sylurus glanis) from the Buško Blato reservoir (Bosnia and Herzegovina). Chemosphere 135:289–296. doi:10.1016/j.chemosphere.2015.04.015
Hontela A (1998) Interrenal dysfunction in fish from contaminated sites: in vivo and in vitro assessment. Environ Toxicol Chem 17:44–48. doi:10.1002/etc.5620170107
Hontela A, Rasmussen JB, Audet C, Chevalier G (1992) Impaired cortisol stress response in fish from environments polluted by PAHs, PCBs, and mercury. Arch Environ Contam Toxicol 22:278–283. doi:10.1007/bf00212086
Hontela A, Daniel C, Rasmussen JB (1997) Structural and functional impairment of the hypothalamo-pituitary-interrenal axis in fish exposed to bleached kraft mill effluent in the St Maurice River, Quebec. Ecotoxicology 6:1–12. doi:10.1023/a:1018699405158
Jarić I, Višnjić-Jeftić Ž, Cvijanović G, Gačić Z, Jovanović L, Skorić S, Lenhardt M (2011) Determination of differential heavy metal and trace element accumulation in liver, gills, intestine and muscle of sterlet (Acipenser ruthenus) from the Danube River in Serbia by ICP-OES. Microchem J 98:77–81. doi:10.1016/j.microc.2010.11.008
Jørgensen LA, Pedersen B (1994) Trace metals in fish used for time trend analysis and as environmental indicators. Mar Pollut Bull 28:24–32. doi:10.1016/0025-326X(94)90182-1
Jovičić K et al (2015) Mapping differential elemental accumulation in fish tissues: assessment of metal and trace element concentrations in wels catfish (Silurus glanis) from the Danube River by ICP-MS. Environ Sci Pollut Res 22:3820–3827. doi:10.1007/s11356-014-3636-7
Kalay M, Canli M (2000) Elimination of essential (Cu, Zn) and nonessential (Cd, Pb) metals from tissue of a freshwater fish Tilapia zillii following an uptake protocol. Tukr J Zool 24:429–436
Karadede-Akin H, Ünlü E (2007) Heavy metal concentrations in water, sediment, fish and some benthic organisms from Tigris River, Turkey. Environ Monit Assess 131:323–337. doi:10.1007/s10661-006-9478-0
Karaytug S, Karayakar F, Ciftci N, Cicik B, Ay O, Erdem C (2010) Effects of Cadmium on Sera Glucose and Cortisol Levels in Clarias gariepinus (Burchell, 1822). J Anim Vet Adv 9:2159–2162. doi:10.3923/javaa.2010.2159.2162
Khan AT, Weis JS (1993) Bioaccumulation of heavy metals in two populations of mummichog (Fundulus heteroclitus). Bull Environ Contam Toxicol 51:1–5. doi:10.1007/bf00200992
Linde AR, Klein D, Summer KH (2005) Phenomenon of hepatic overload of copper in Mugil cephalus: role of Metallothionein and patterns of copper cellular distribution. Basic Clin Pharm Toxicol 97:230–235. doi:10.1111/j.1742-7843.2005.pto_56.x
Łuszczek-Trojnar E, Drąg-Kozak E, Popek W (2013) Lead accumulation and elimination in tissues of Prussian carp, Carassius gibelio (Bloch, 1782), after long-term dietary exposure, and depuration periods. Environ Sci Pollut Res Int 20:3122–3132. doi:10.1007/s11356-012-1210-8
Randall DJ, Brauner CJ, Thurston RV, Neuman JF (1996) Water chemistry at the gill surfaces of fish and the uptake of xenobiotics. In: Taylor EW (ed) Toxicology of aquatic pollution: physiological, molecular and cellular approaches. Cambridge University Press, Cambridge, pp 1–16. doi:10.1017/CBO9780511735516.002
Reid SD, McDonald DG (1991) Metal binding activity of the gills of Rainbow Trout (Oncorhynchus mykiss). Can J Fish Aquat Sci 48:1061–1068. doi:10.1139/f91-125
Shah AQ et al (2009) Accumulation of arsenic in different fresh water fish species—potential contribution to high arsenic intakes. Food Chem 112:520–524. doi:10.1016/j.foodchem.2008.05.095
Shinn C, Dauba F, Grenouillet G, Guenard G, Lek S (2009) Temporal variation of heavy metal contamination in fish of the river lot in southern France. Ecotoxicol Environ Saf 72:1957–1965. doi:10.1016/j.ecoenv.2009.06.007
Thang NQ, Phuong NTK, Van Tan L (2017) Endocrine stress response in Oreochromis sp. from exposure to waterborne cadmium: the plasma cortisol analysis. Toxicol Environ Chem 99:285–293. doi:10.1080/02772248.2016.1172583
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591
Wong CK, Wong PPK, Chu LM (2001) Heavy metal concentrations in marine fishes collected from fish culture sites in Hong Kong. Arch Environ Contam Toxicol 40:60–69. doi:10.1007/s002440010148
Zaki MS, Fawzi OM, Moustafa S, Seamm S, Awad I, El-Belbasi HI (2009) Biochemical and Immunological studies in Tilapia Zilli exposed to lead pollution and climate change. Nat Sci 7:90–93
Zare S, Afaghi A, Heidari R, Asadpoor Y, Shiri S (2007) Effects of Lead Nitrate (PbNO3) on the glucose and cortisol hormone levels in Common Carp, Cyprinus carpio. Pak J Biol Sci 10:2587–2590. doi:10.3923/pjbs.2007.2587.2590
Acknowledgements
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2014R1A1A4A01008793).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Thang, N.Q., Huy, B.T., Van Tan, L. et al. Lead and Arsenic Accumulation and Its Effects on Plasma Cortisol Levels in Oreochromis sp.. Bull Environ Contam Toxicol 99, 187–193 (2017). https://doi.org/10.1007/s00128-017-2113-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2113-7