Abstract
The effect of untreated olive mill wastewater (OMW) spreading on chemical and biological soil properties of two different fields located in Campania (Italy) was investigated. Fields were irrigated since 2003 with quantities of about 30 m3 ha−1 year−1, a volume lower than the maximum limit of 80 m3 ha−1 year−1 established by Italian law. Results showed that the addition of OMW, even if repeated for many years, had little impact on pH, electrical conductivity, organic matter, concentrations of main cations and polyphenolic content of both soil plots; moreover, microbial respiration was low during the winter time, but an increase was evident in the second sampling carried out in warm season. This study suggests that OMW, without pre-treatments, can be annually used for crops and tree irrigation. As a consequence, OMW should be a readily and inexpensive source of nutrients that could replace chemical fertilizers which are extensively employed in agricultural practices of Mediterranean countries.
Similar content being viewed by others
Olive oil production is highly important for the economy, ecology and social life of many Mediterranean countries such as Spain, Italy, and Greece, which account for about 75 % of olive oil world production (Doula et al. 2012). However, the extraction process generates a high amount of olive mill wastewaters (OMW), with an average annual production of 30 million m3 (Mekki et al. 2013). This waste presents high values of chemical oxygen demand (COD, 50–180 g L−1) and biochemical oxygen demand (BOD5, 40–95 g L−1), phytotoxic properties, and resistance to biodegradation due to the high polyphenol and organic content (Mekki et al. 2007; Rinaldi et al. 2003). OMW cannot directly enter sewage systems because it could alter the efficiency of wastewater treatment plants, due to the high concentrations of polyphenols, long chain fatty acids, and metals. Microorganisms, insects, larvae and earthworms, naturally present in the soil, are able to metabolize OMW compounds and produce a mixture of complex aromatic molecules known as humic and fulvic acids, which improve soil fertility (Niaounakis and Halvadakis 2004). It is important to note that negative effects might occur if a high amount of OMW was disposed on soil, because of its high salinity, low pH, and the presence of toxic biodegradable substances (Zenjari and Nejmeddine 2001).
Italy was the first European country to establish specific laws for the disposal of mill wastes on soil. The Italian Law no. 574/1996, together with the Ministry Decree 6 July 2005, defines the maximum amount of OMW tolerated on fields as 80 and 50 m3 ha−1 year−1 when obtained by continuous (centrifuge extraction system) and traditional (press extraction system) processes, respectively. To help resolve the chemical complexity of OMW, different procedures such as aerobic treatment, anaerobic digestion, and composting have been proposed (Mekki et al. 2013).
Some studies indicated a positive effect of OMW on physical, chemical and microbiological properties of soil (Pagliai et al. 2001). Ayoub et al. (2014) and Chaari et al. (2014) reported OMW spreading had beneficial effects on top soil, such as nutrient availability for plant growth. Conversely, Mekki et al. (2006) found that OMW caused negative changes in microbial soil properties, decreasing or inhibiting microflora growth. Zenjari and Nejmeddine (2001) established OMW spreading compromised soil fertility, altering physical and chemical soil properties.
Only recently have studies evaluated the long-term effect of OMW disposal on soil properties (Chaari et al. 2015; Kavvadias et al. 2014). Chaari et al. (2015) observed a positive soil effect due to the supply of organic matter (OM) and macronutrients, but when a high dose (200 m3 ha−1 year−1) was provided for nine successive years, a cumulative effect of soil salinization became evident. Kavvadias et al. (2014) highlighted the effect on soil after 11 years of disposal of raw OMW. They reported that the uncontrolled and elevated disposal resulted in a source of pollution mainly on the soil surface, concluding it was necessary to establish soil quality standards in order to identify soils well-suited to OMW spreading.
Current research assesses the chemical and biological properties of soils after treatment with raw OMW. The study was performed on two different soils, located in Avellino province (Italy), that have been irrigated for 11 years with a low volume of OMW (30 m3 ha−1 year−1), corresponding to the annual OMW production of the factory mill. Soils were analyzed after the last spreading performed in October 2013.
Materials and Methods
Two fields belonging to Basso Fedele and Figli S.r.l. are situated in San Michele di Serino (40°53′00″N, 14°51′00″E-AV, Italy) and in Castelfranci (40°55′56″N, 15°02′39″E-AV, Italy) and are normally cultivated with vineyards. The area has a Mediterranean temperate climate with a mean rainfall of 775 mm year−1, and average daily temperatures range between 14.5 and 28.7°C in summer and 3.1 and 10.5°C in winter.
Each field was divided into two parts: a control (no OMW treatment) and treatment with OMW (Table 1). Since 2003, a quantity of 30 m3 ha−1 of OMW was distributed annually in autumn on fields with an automatic tanker. Soil sampling layers (0.3 m) from the control and treated areas were collected in February 2014 (winter) and June 2014 spring).
All sampling and analyses were done according to the official directives of the Italian Ministry of Agriculture and Forestry (G. U. no. 248-21/10/1999). Soils were stored at 4°C until analysis. Each sample (20 g) was resuspended in 50 mL of deionized water, incubated for 2 h at 25°C, and extracts were recovered by centrifugation. Soil electrical conductivity (EC) and pH were measured by a conductivity-meter (Eutech Instruments Oakton) and pH-meter (Mettler Toledo), respectively.
Dry weight and moisture contents were determined by weighing samples before and after drying overnight at 105°C in pre-weighed porcelain dishes. OM was determined after placing samples in a muffle furnace and burned at 550°C for 4 h. After being cooled in desiccators, samples were re-weighed and OM was expressed as a percentage.
Total organic nitrogen was determined by NH3 distillation after Kjeldahl digestion, according to the ISO 1126:1995 method. Briefly, 10 mL of concentrated H2SO4 and 1 tablet of catalyst (K2SO4/Cu5O4/Se powder-100/10/1) were added to 0.5 g of each soil. Samples were digested at 315°C for 4–5 h, and the products were automatically distilled and titrated by Kjeldahl auto-analyzer. Results were calculated on dry mass of soil and expressed in g kg−1.
Analyses of Ca, Na, Mg, K, Fe, As, Cd, Cr, Cu, Pb, and Zn were performed by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES, Optima2000, PerkinElmer, Italy), equipped with an axially viewed torch, Scot type spray chamber and charge coupled device detector (CCD). A hot digestion of samples was carried out with HF and HClO4 and, after cooling, samples were filled up to 50 mL with deionized water. ICP-OES data were collected and reprocessed using WinLab32 Software (Perkin-Elmer). Operating parameters were the following: argon and carrier gas flows were set at 15 and 1.0 mL min−1, respectively; the auto-integration times, the spectral range and the spectral lines were chosen according to Perkin Elmer specification methods; samples were taken up at a flow rate of 1.5 mL min−1. The multi-element standard solution was used for five-point calibration curves; concentration ranges of the working solutions were 0.5–10 mg L−1 for Na, Ca, K and Mg; 100–2000 μg L−1 for Al, Pb and Zn; 50–1000 μg L−1 for Cu and 20–400 μg L−1 for As, Cr and Fe.
OMW was analyzed to determinate its polyphenolic content by the Folin-Ciocalteu assay (Singleton and Rossi 1965). Variable volumes (10, 20 and 30 µL) of OMW were mixed with 750 µL of Folin–Ciocalteu reagent and 600 µL of 7.5 % (w/v) Na2CO3. Absorbance was measured at 765 nm and polyphenol amount was calculated by a calibration curve using a gallic acid standard. Results were expressed as mg of gallic acid equivalent (GAE) per mL of OMW. Total phenolic content of soils was determined similarly as previously described for OMW and was expressed as mg of GAE per g of soil analyzed.
Determination of ortho-diphenols on soils was achieved using the Arnow assay (Arnow 1937), with three different solutions of 0.5 M HCl, 1.45 M NaNO2–0.4 M Na2MoO4 and 1.0 M NaOH. Sample absorbance was measured at 500 nm and the ortho-diphenolic acid content was determined by a calibration curve obtained using caffeic acid as standard. Results were expressed as mg of caffeic acid equivalent (CAE) per g of soil analyzed.
Potential microbial activity was assessed by the respiration assay, measuring the oxygen consumption. Briefly, 2.5 g of soil were put into a batch containing 25 mL of 0.9 % NaCl sterile solution. After stirring for 30 min, 3 mL of soil suspension were tested at 28°C for its biological activity, measuring the oxygen consumption by an oxygen monitor (YSI mod. 240/B) adding 1 % glucose. Results were expressed as µmoles of O2 h−1 g−1 of soil.
All data were expressed as mean ± standard deviation (SD) of n = 3 determinations. Student t test with a 0.05 and 0.01 significance levels were used with control and treatment parameters (Statgraphics Plus software 4.0-Statpoint Technologies, Inc.).
Results and Discussion
Divergent outcomes were reported in literature of the direct OMW disposal influence on soil properties (Chaari et al. 2014; Mekki et al. 2006), as well its use as a source of OM and nutrients. Control soils from San Michele di Serino and Castelfranci were analyzed to evaluate if they were appropriate for OMW spreading (Ministry Decree of Italy, 13/09/99). Soils were silty-clay, slightly calcareous with excessive active limestone and scarce in OM and N (Table 2). The field in San Michele di Serino was poor in exchangeable Mg, but rich in exchangeable K. The Castelfranci field was characterized by high levels of exchangeable Mg, but low amounts of exchangeable K.
The pH and EC values of control and treated soils in San Michele di Serino and in Castelfranci, collected in February and June after 11 annual OMW treatments, are shown in Table 3. The pH ranged from 7.60–8.10 after 4 months from last spreading, to 8.24–8.42 after 8 months, for all samples. This slight increase of pH recorded at 8 months could be explained considering the mineralization of C, that naturally occurs in the same way in control and treated soil samples. As a result, the buffering capacity of the soil counterbalances the acidity of OMW, mainly due to the presence of organic acids. Similar results were obtained by Chaari et al. (2015) after a regular application of higher doses in a 9 years study.
Conductivity was unaltered after spreading in both treated soils, if compared with the controls (Table 3). The EC value in the soils ranged between 180 and 220 µS and remained below the salinization threshold of 4000 µS (Chaari et al. 2014), thus concluding that OMW treatments had no negative effect on EC of the two soils.
Soil moisture varied between 5.19 % and 7.26 % in all samples collected in February, as shown in Table 4. The four soils analyzed in June exhibited a reduction of water content, ranging between 3.88 % and 5.89 %, which could be a natural decrease due to higher temperatures recorded during the dry months.
OM content from both sites, for all samples collected in June did not significantly increase compared to previous values from February, ranging from 0.50 %–1.09 % to 0.75 %–1.21 % (Table 4). Moreover, OM percentages were unchanged both in control and treated fields, concluding that the spreading treatment does not cause variations on soil properties. It was possible that added organic carbon from OMW treated fields was degraded biologically by aerobic and anaerobic microorganisms very quickly and therefore, already in February, soils in San Michele di Serino and in Castelfranci displayed the same chemical properties of the control fields. Moreover, OM content of both sites, control and treatment, showed a decrease over time (Tables 2, 4). In spite of the application, OMW spreading was not sufficient to prevent OM diminution in the treated soils, probably due to the low quantities added.
For their life cycle, plants and crops must absorb macronutrients and essential micronutrients. Soil macronutrients content of N, Ca, Na, Mg, and K, 4 and 8 months after the last OMW treatment, were listed in Table 5.
Nitrogen content, which ranged from 0.52 to 1.45 g kg−1 in February, decreased to 0.17–0.54 g kg−1 in June. This diminution could be due to the element uptake by microorganisms and plants’ active metabolism. In general, the input of nutrients by OMW treatment was not evident since, just after 4 months from the last spreading, there was not an increase of nutrients in treated soils compared with control soils. In spite of the high concentration of K in OMW (Niaounakis and Halvadakis 2004), its level in both control and treated soils remained low. The average contents of Ca, Na, Mg, and K described the same behavior depicted above for N in all soil samples, as shown in Table 5, thus concluding that macronutrients were not affected by OMW dispersion.
As reported by Chaari et al. (2014), the ideal cation ratios recommended for plant growth are Ca/Mg 6.5/1; Ca/K 13/1; and Mg/K 2/1. Cation rates, calculated from values shown in Table 5, were lower. The Ca/Mg ratio was from 1.49/1 in Castelfranci-C to 4.87/1 in San Michele di Serino-T; the Ca/K ratio was from 1.06/1 in Castelfranci-C to 2.23/1 in Castelfranci-T; the Mg/K ratio was from 0.33/1 in San Michele di Serino-T to 0.89/1 in Castelfranci-T. For almost all soils, ratios were higher in treated than controls, thus suggesting OMW disposal might improve soil fertility. Soil mineral composition and its cation exchange capacity are affected by many variables like soil texture (number of negative charges linked to humus and clay), crop rotation, pH, and OM (Rehm 1994).
Metal contents of soils were also analyzed. Results in Table 6 showed low concentrations of Cu, Fe, Zn, As, Pb, Cd, and Cr in all samples. Arsenic and Pb values were below limits of detection of 14 and 10 µg kg−1, respectively. Cadmium and Cr concentrations were below the Italian law limits of 3 and 150 mg kg−1, respectively. Furthermore, Cu, Fe, and Zn, essential nutrients for plant growth (Wuana and Okieimen 2011), were below the Italian law limits of 120, 200 and 150 mg kg−1, respectively. The average concentration of Cr, Cu, Fe, and Zn was almost always greater in control than in treated soils. Metal contents can be influenced by chemical properties such as pH, OM content and soil texture. These combined features could favor metal retention or mobilization and affect metal bio-availability (Madrid 1999).
The assessment of polyphenol concentration in soil is considered difficult and with high degree of uncertainty, due to the lack of a generally accepted threshold. According to average Dutch standards, soils with values >40 mg kg−1 are considered contaminated (Kavvadias et al. 2014; Swartjes 1999). Initial polyphenolic content of OMW was 0.96 ± 0.02 mg GAE mL−1. This was lower than those reported by Chaari et al. (2014) (4.2 mg mL−1); Mekki et al. (2013) (8.6 mg mL−1); Mekki et al. (2006) (8.3 mg mL−1); and Zenjari and Nejmeddine (2001) (3.5 mg mL−1).
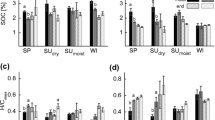
Total phenols and ortho-diphenols were determined for control and treated soils in both fields (Fig. 1). Total phenols varied from 1.01 to 1.27 μg g−1, after 4 months from the last OMW application, and all values remained constant in June, between 1.01 and 1.35 μg g−1. The same trend was reported for ortho-diphenols. They ranged from 0.72 to 0.99 μg g−1 in February, and they remained constant in June, from 0.71 to 0.77 μg g−1. Polyphenolic fraction could be quickly degraded and transformed into humic substances and, after just 4 months, the values were the same obtained for the controls. These results were consistent with data obtained 8 months after the last OMW application.
OMW spreading usually causes a rapid decrease of the total microflora, due to the presence of bacteriostatic or bactericidal compounds (Mekki et al. 2006; Pagliai et al. 2001). This reduction takes place in the days immediately following the treatment (1 or 2 weeks), but microbial activity usually increases after 1 month. Soil respiration is an index of the potential metabolic activity of soil biota. The process is linked to fertilization but it is also affected by temperature and humidity of soil.
Results in Table 7 showed low microbial respiration due to the fact that samples were collected between two rows of a vineyard, far from the rhizosphere, which is an environment favourable to microorganisms. A slight increase in respiration activity of the microbial biomass was recorded during the Spring when temperature increased. A similar level of OMW (36 m3 ha−1 year−1) was supplied by Saadi et al. (2007) in a short-term effect study. They reported OMW stimulated soil microbial activity and was not harmful to soil microflora.
In conclusion, this work established the spreading of OMW does not negatively affect soil properties. In particular, no significant differences in the chemical and biological properties between control and treated soils were evident when the quantity distributed was lower than the maximum limit of 80 m3 ha−1 year−1 stated by Italian law. Results confirmed OMW, without any previous treatments such as aerobic or anaerobic digestion, could be useful as an available and cheap amendment to irrigation water for crops and trees. At the same time, its spreading may offer the opportunity to solve environmental issues linked to wastewater management.
References
Arnow LE (1937) Colorimetric determination of the components of 3,4-dihydroxyphenylalaninetyrosine mixtures. J Biol Chem 118:531–537
Ayoub S, Al-Absi K, Al-Shdiefat S, Al-Majali D, Hijazean D (2014) Effect of olive mill wastewater land-spreading on soil properties, olive tree performance and oil quality. Sci Hortic 175:160–166
Chaari L, Elloumi N, Mseddi S, Gargouri K, Bourouina B, Mechichi T, Kallel M (2014) Effects of olive mill wastewater on soil nutrients availability. Int J Interdiscip Multidiscip Stud 2:175–183
Chaari L, Elloumi N, Mseddi S, Gargouri K, Rouina BB, Mechichi T, Kallel M (2015) Changes in soil macronutrients after a long-term application of olive mill wastewater. J Agric Chem Environ 4:1–13
Doula MK, Tinivella F, Moreno Ortgego LL, Kavvadias VA, Sarris A, Theocharopoulos S, Sanchez-Monedero MA, Elaiopoulos K (2012) Good practices for the agronomic use of olive mill wastes. Application Guide published in project “PROSODOL: Strategies to improve and protect soil quality from the disposal of olive oil mills’ wastes in the Mediterranean region” LIFE07 ENV/GR/000280, Edited by Maria K Doula, pp 1–61
Kavvadias V, Doula M, Theocharopoulos S (2014) Long-term effects on soil of the disposal of olive mill waste waters (OMW). Environ Forensics 15:37–51
Madrid L (1999) Metal retention and mobility as influenced by some organic residues added to soils: a case study. In: Selim HM, Iskandar IK (eds) Fate and transport of heavy metals in the vadose zone. Lewis Publishers, Boca Raton, pp 201–223
Mekki A, Dhouib A, Sayadi S (2006) Changes in microbial and soil properties following amendment with treated and untreated olive mill wastewater. Microbiol Res 161:93–101
Mekki A, Dhouib A, Sayadi S (2007) Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. J Environ Manag 84:134–140
Mekki A, Dhouib A, Sayadi S (2013) Review: effects of olive mill wastewater on soil properties and plant growth. Int J Recycl Org Waste Agric 2:15
Niaounakis M, Halvadakis CP (2004) Olive-mill waste management: literature review and patent survey. Typothito - George Dardanos Publications, Athens
Pagliai M, Pellegrini S, Vignozzi N, Papini R, Mirabella A, Piovanelli C, Gamba C, Miclaus N, Castaldini M, De Simone C, Pini R, Pezzarossa B, Sparvoli E (2001) Influenza dei reflui oleari sulla qualità del suolo. Supplemento a L’Informatore Agrario 50:13–18
Rehm G (1994) Soil cation ratios for crop production. North Central Regional Extension Publication 533. www.extension.umn.edu/distribution/cropsystems/DC6437.html
Rinaldi M, Rana G, Introna M (2003) Olive-mill wastewater spreading in southern Italy: effects on a durum wheat crop. Field Crops Res 84:319–326
Saadi I, Laor Y, Raviv M, Medina S (2007) Land spreading of olive mill wastewater: effect on soil microbial activity and potential phytotoxicity. Chemosphere 66:75–83
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Swartjes F (1999) Risk-based assessment of soil and groundwater quality in the Netherlands: standards and remediation urgency. Risk Anal 19:1235–1249
Wuana RA, Okieimen FE (2011) Heavy metal in contaminated soils: a review of sources, chemistry, risk and best available strategies for remediation. ISRN 2011:1–20
Zenjari B, Nejmeddine A (2001) Impact of spreading olive mill wastewater on soil characteristics: laboratory experiments. Agronomie 21:749–755
Acknowledgments
This work was supported by the project: “Qualità delle produzioni tipiche campane ed il suo territorio: approcci innovativi ed integrati per rafforzare la competitività del sistema Agroalimentare-QUARC”, funded by POR-FESR Campania 2007–2013 (Italy). Thanks are due to Sig. Agostino Navarro for his valuable assistance in sample collection and management. Special thanks are also extended to the kindness welcome of the Basso factory staff.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vella, F.M., Galli, E., Calandrelli, R. et al. Effect of Olive Mill Wastewater Spreading on Soil Properties. Bull Environ Contam Toxicol 97, 138–144 (2016). https://doi.org/10.1007/s00128-016-1830-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1830-7