Abstract
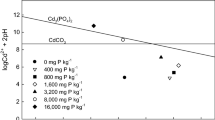
The objective of this study was to determine soil pH conditions that allow cadmium (Cd) to precipitate as Cd minerals in phosphate (P) amended soil. Cadmium immobilization could be attributed primarily to Cd adsorption due to increase in pH and negative charge. Soil pH might not affect Cd precipitation as Cd3(PO4)2 by direct reaction of Cd and P in the studied soil, even when soil pH increased up to 9.0. However, Cd might precipitate as CdCO3 with increasing pH up to 9.0 in P untreated soil and up to 8.0 in P treated soil depending on CO2 level.


Similar content being viewed by others
References
Biling W, Zhengmiao X, Jianjun C, Juntao J, Qiufeng S (2008) Effects of field application of phosphate fertilizers on the availability and uptake of lead, zinc and cadmium by cabbage (Brassica chinensis L.) in a mining tailing contaminated soil. J Environ Sci 20:1109–1117
Bolan NS, Adriano DC, Duraisamy P, Mani A, Arulmozhiselvan K (2003) Immobilization and phytoavailability of cadmium in variable charge soils: I effect of phosphate addition. Plant Soil 250:83–94
Cavallaro N, McBride MB (1978) Copper and cadmium adsorption characteristics of selected acid and calcareous soils. Soil Sci Soc Am J 42:550–556
Chen S, Xu M, Ma Y, Yang J (2007) Evaluation of different phosphate amendments on availability of metals in contaminated soil. Ecotoxicol Environ Saf 67:278–285
Harris DC (1995) Quantitative chemical analysis, 4th edn. W.H. Freedman and Company, New York
Hong CO, Chung DY, Lee DK, Kim PJ (2010) Comparison of phosphate materials for immobilizing cadmium in soil. Arch Environ Contam Toxicol 58:268–274
Jalali M, Khanlari ZV (2008) Effect of aging process on the fractionation of heavy metals in some calcareous soils of Iran. Geoderma 143:26–40
Lide DR (2006) CRC handbook of chemistry and physics, 87th edn. CRC Press, Inc., Boca Raton, FL, p 299
Lieu VT, Kalbus GE (1988) Potentiometric titration of acidic and basic compounds in household cleaners. J Chem Educ 65:184
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York
Lu A, Zhang S, Shan X (2005) Time effect on the fractionation of heavy metals in soils. Geoderma 125:225–234
McBride MB (1980) Chemisorption of Cd2+ on calcite surfaces. Soil Sci Soc Am J 44:26–28
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York, p 330
McGowen SL, Basta NT, Brown GO (2001) Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J Environ Qual 30:493–500
ME (Ministry of Environment, Republic of Korea) (2005) The Korean Soil Environmental Conservation Act, ME, Gwacheon, South Korea, p 58 (in Korean)
Naidu R, Bolan NS, Kookana RS, Tiller KG (1994) Ionic strength and pH effects on the adsorption of cadmium and the surface charge of soils. Eur J Soil Sci 45:419–429
Rajaie M, Karimian N, Maftoun M, Yasrebi J, Assad MT (2006) Chemical forms of cadmium in two calcareous soil textural classes as affected by application of cadmium-enriched compost and incubation time. Geoderma 136:533–541
SAS Institute Inc. (2001) User’s guide: statistics SAS Version 8.2. SAS Institute, Cary, NC
Schofield RK (1949) Effect of pH on electric charges carried by clay particles. J Soil Sci 1:1–8
Sparks DL (1996) Methods of soil analysis part 3 chemical methods. Soil Science Society of America Inc., American Society of Agronomy Inc., pp 1146–1155
Sparks DL (2003) Environmental soil chemistry. Academic Press, San Diego, pp 92–94
Street JJ, Sabey BR, Lindsay WL (1978) Influence of pH, phosphorus, cadmium, and sewage sludge and incubation time on the solubility and plant uptake of cadmium. J Environ Qual 7:286–290
Su C, Tie-heng S, I-na LS, Qi-xing Z, Lei C (2006) Influences of phosphate nutritional level on the phytoavailability and speciation distribution of cadmium and lead in soil. J Environ Sci 18:1247–1253
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ009140)” Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hong, C.O., Owens, V.N., Kim, Y.G. et al. Soil pH Effect on Phosphate Induced Cadmium Precipitation in Arable Soil. Bull Environ Contam Toxicol 93, 101–105 (2014). https://doi.org/10.1007/s00128-014-1273-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1273-y