Abstract
A rapid, sensitive and highly specific HPLC–MS/MS method with direct on-line preparation was applied for the determination of 20 common pharmaceuticals in hospital and urban wastewater. Median drug concentrations were quite similar in the majority of samples, cerca 1 μg L−1 ranging from 0.06 to 2.67 μg L−1 in both water. Pharmaceutical hospital contribution, below 1 %, was negligible, as compared to the huge amount in the municipal plant flow. Due to only partial elimination in the plant, hundreds of kilograms of harmful waste per year are discharged in the River Seine. Therefore, to reduce potential human and environmental exposure, a topic of major concern, an efficient drug treatment procedure should be used at the municipal plant stage in order to reduce urban wastewater pollution. The HPLC–MS/MS method could be a very useful tool to optimize the pharmaceutical wastewater treatment process.
Similar content being viewed by others
In a previously reported study, we underlined the importance of anthropogenic metals in hospital and urban wastewater (Goullé et al. 2012). In this present paper we report our recent findings regarding the presence of drugs in both wastewaters. Large quantities of pharmaceuticals are used throughout the world each year in human or veterinary medical prescriptions. A variable proportion of drugs and/or metabolites is present in wastewater and then released into the environment. The presence of pharmaceuticals has been reported not only in surface but also in ground and sometimes tap water (Fatta-Kassinos et al. 2011; Escher et al. 2011). These contaminants remain a topic of major concern as some are endocrine disruptors (Li et al. 2010; Ge et al. 2010), while others are responsible for antibiotic resistance (Rowan 2011; Le-Minh et al. 2010). In fact for many contaminants the risk assessment evaluation is difficult. Moreover their principal effect on human health effect still remains poorly understood (Escher et al. 2011). Human, animal urine and feces including not only drugs or metabolites, but also pure pharmaceuticals and drugs of abuse (Verlicchi et al. 2012, Baker and Kasprzyk-Hordern 2011), pesticides (Tadeo et al. 2010) metals (Goullé et al. 2012), and various chemicals are eventually directly or indirectly discharged into the wastewater (Pal et al. 2010). To assess the environmental load of these substances, we evaluated the concentration of metals (Goullé et al. 2012), as well as pharmaceuticals commonly present in different wastewaters. This in depth study is based on a major research program supported by grants from the Regional Heath Department of Upper-Normandy—France, and the main drug results are presented in our study. Representative samples of wastewater from the Rouen University Hospital (2,500 beds) and from Rouen Treatment Plant were analyzed. Hospital wastewater (HWW) is, in fact, initially discharged without any specific treatment of their pollutants into the municipal network wastewater (an urban area of 400,000 inhabitants). Recently Baranovska and Kowalsi (2012) published a rapid UHPLC method, for the simultaneous determination of drugs in surface water and wastewater. As our research group has a particularly high expertise not only in HPLC–MS/MS drug and drug of abuse analysis, but also in high speed automated on-line preparation, we conducted an exhaustive study regarding the presence of pharmaceuticals in wastewater (Lacroix et al. 2008). The aim of this study was:
-
to apply a very fast, sensitive and highly specific on-line HPLC–MS/MS technique that we have previously developed for routine drugs and drugs of abuse determination in biological fluids (Lacroix et al. 2008);
-
to quantify twenty pharmaceuticals and metabolites in Hospital and urban wastewater with this method: acebutolol, bisoprolol, buflomedil, carbamazepine, celiprolol, ceterizine and hydroxyzine metabolite, citalopram, codeine, disopyramide, flecainide, labetalol, lidocaine, meprobamate, metoprolol, oxazepam, propoxyphen and metabolite norpropoxyphen, propranolol, tramadol, venlafaxine and metabolite desmethylvenlafaxine, verapamil;
-
to evaluate the total annual load of these drugs into the environment via the River Seine, after they are discharged from the treatment plant.
Materials and Methods
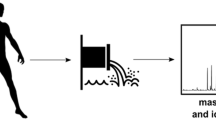
During 29 days, from April the 16th to May the 14th four wastewater collections were performed each day, with samplers connected to the flow. These samples were all proportional continuous samples. The part of the flow collected was exactly three parts per 1,000, therefore the samples collected each day were entirely representative of the 24 h period at each site: hospital outlet (HWW); Treatment Plant (TPWW): inlet part, to assess the dilution effect of HWW at the entrance of the plant; middle part, after primary physicochemical treatment; outlet part, after the secondary biological treatment and just prior to water discharge into the environment i.e. the River Seine (Fig. 1).
The HWW was not affected by rainfall events, as this water was collected separately; it was mainly tap water, shower water and patient excreta. In contrast, variations of the treatment plant flow were observed during rainfalls as rain water was also collected together with all the wastewater entering the treatment plant. The hydraulic residence duration in the treatment plant was 27 h, so we considered the retention offset of 1 day between TPWW inlet and TPWW outlet for statistics. During the 29 day period, 19 were working days, 10 were non-working days i.e. four Saturdays, four Sundays, plus two non-working days. The column-switching LC–MS/MS system consisted of an 1525 Micro Binary Pump (pump 1 for wash solvent; Waters, Milford, MA), an Alliance 2795 HPLC pump (pump 2 for elution mobile phase; Waters, Milford, MA), a sample injection valve with 350 μL sample loop (Rheodyne, Cotati, CA), a 10-port valve allowing switching or direct injection (valve 1; Rheodyne), a six-port switching valve (valve 2; Rheodyne), and a Quattro Micro™ API tandem mass spectrometer (Waters, Milford, MA) equipped with an atmospheric pressure ionization (API) electrospray ionization interface (ESI) and controlled by computer through MassLynx software (Version 4.1). An Oasis HLB (2.1 × 20 mm; 25 μm, Waters, Milford MA) was used as an extraction column and Atlantis C18 (2.1 × 150 mm; 3 μm, Waters, Milford MA) as a reversed-phase analytical column. A 350 μL wastewater sample was applied on the extraction column. Then, wash solvent for sample extraction and enrichment was delivered by pump 1 at 2 mL min−1, and Milli-Q water fortified with 0.2 % ammonia. After the extraction phase on the Oasis HLB column, the mobile phase for sample elution and separation, delivered by pump 2 at 0.3 mL min−1, consisted of 20 mM ammonium formate aqueous solution adjusted to pH 2.8 with formic acid (solvent A) and acetonitrile/solvent A (90:10, v/v) [solvent B] was applied on the Atlantis analytical column for elution and separation of the pharmaceuticals. Analysis cycle time including SPE and chromatographic separation was 25 min. Detection was operated in the positive ionization mode. The probe capillary voltage was 3.2 kV; the source block and desolation temperatures were 120 and 450°C, respectively. Cone voltages, collision energies, have been described elsewhere (Lacroix et al. 2008). Quantitative measurements were carried out by LC–MS/MS in the multiple reaction monitoring (MRM) mode.
The statistical analysis of the data was based on the fact that wastewater samples, collected daily at each site (HWW output, TPWW input, TPWW middle and TPWW output) with samplers connected to the flow, were representative for each 24 h period. Therefore, for each day and each site, the average concentration and total amount could subsequently be deduced for each drug. An estimate of the total amount eliminated during the experimental period of 29 days could be deduced. The statistical treatment of the data was performed with the R language and environment for statistical computing (http://www.R-project.org).
Results and Discussion
As previously mentioned, it is important to underline that the part of the wastewater collected at the four sites was exactly three parts per 1,000 of the total flow, therefore all the samples were entirely representative of the flow at the site where they were collected, so the total amount eliminated for each drug could be quantitated during the 29 day period. The raw wastewater after centrifugation, and filtration (0.7 μm), was a matrix very similar to urine. Regarding the quality control urine results, all the Z scores obtained were excellent, for the pharmaceuticals including: codeine, meprobamate, oxazepam, venlafaxine. An external aqueous calibration curve was used that produce exactly the same results as a standard addition curve (Lacroix et al. 2008). The main modification compared with the previous reported method was a 350 μL sample loop size for drugs in wastewater instead of a 100 μL sample loop size for drugs in serum, plasma or whole blood. The limit of detection ranged from 2 μg L−1 for propranolol to 41 μg L−1 for citalopram. The direct on-line preparation stage and the LC–MS/MS coupling technique can offer many considerable benefits:
-
it avoids a very tedious and time consuming extraction step, a few minutes, instead of 2 h per sample;
-
the sample size of 0.35 mL is greatly reduced as regards usual volumes ranging from 60 to 1,000 mL;
-
the high sensitivity and specificity of the multidrug LC–MS/MS method.
Recently Baranovska and Kowalsi (2012) published a very interesting article in this journal regarding UHPLC with UV detection, using a SPE pre-concentration stage. However, this type of procedure is not only time-consuming, but also normally uses a very large sample of water, one liter. In contrast, the proposed method is very fast, as the SPE on-line extraction allows a direct pre-concentration stage. Furthermore, as the HPLC–MS/MS technique is very sensitive and highly specific, we were able to reduce the sample to 0.35 mL without sensitivity loss. As evidence, some analgesics: tramadol, codeine and propoxyphen; but also psychotropics: meprobamate, oxazepam and venlafaxin; and cardiotropic drugs: celiprolol, flecainide; among the most frequently prescribed were eliminated in the μg L−1 range (Table 1). For the six higher concentrations, the daily median HWW and TPWW inlet values during the 29 day period were respectively: celiprolol 0.5 and 2.7 μg L−1; tramadol, 2.4 and 1.1 μg L−1; meprobamate, 1.6 and 1.2 μg L−1; propoxyphen, 0.7 and 1.4 μg L−1; oxazpam, 1.1 and 1.2 μg L−1; flecainide, 0.5 and 1.0 μg L−1; venlafaxine, 0.8 and 0.8 μg L−1; codeine, 0.7 and 0.5 μg L−1 (Table 1). These findings were in accordance with those previously reported in recent reviews (Deblonde et al. 2011; Verlicchi et al. 2012). As the samples collected were representative of the total flow at each location, due to proportional continuous samples, the median daily amount of each drug could be accurately measured during the 29 day period. For the six highest quantities of pharmaceuticals entering the TPWW: celiprolol, propoxyphen, meprobamate, oxazepam, tramadol, flecainide, the daily median amounts ranged from 0.1 to 0.7 g in HWW, and they were in the same range from 77 to 225 g in the TPWW inlet and in the TPWW outlet. The HWW pharmaceutical waste contribution into the plant was low, i.e. below 0.5 % for all drugs except for tramadol (0.8 %). This could be explained by the fact that the drug mean concentration was rather similar in the HWW and in the TPWW inlet, but as the HWW mean flow was 289 m3 over the 29 day period and the TPWW mean inlet flow was 81,798 m3 during the same period, the HWW mean flow was only 0.35 % of the TPWW inlet. These results were totally different from those we obtained with metals: silver and gadolinium used in radiodiagnostic and antineoplastic platinum chemotherapy. The hospital contribution of these elements into the plant was 5.3 % and 9.4 % for gadolinium and platinum respectively (Goullé 2012). Based on the assumption that the period was representative of the whole year, the total annual amount of pharmaceuticals released into the environment was hundreds of kilograms i.e. celiprolol 75.6 kg, tramadol 38.0 kg, oxazepam 31.9 kg, flecainide 28.2 kg, venlafaxine 24.3 kg (Table 1). The plant removal percentage was negligible, ranging from zero to 7.8 % for these drugs (Table 1).
In contrast, removal percentages for buflomedil, labetalol, meprobamate, propoxyphen, verapamil and acebutolol in the treatment plant were very significant, ranging from 52 % to 75 %. Furthermore as regards celiprolol, the drug amount eliminated per year in the River Seine for this urban area was 75.6 kg, which is of major concern for the environment. This underlines the substantial variation in efficacy to eliminate pharmaceuticals in the treatment plant as previously reported (Deblonde et al. 2011; Le-Minh et al. 2010; Pal et al. 2010; Verlicchi et al. 2012). The on-line HPLC–MS/MS method which is very fast, sensitive and highly specific, has been successfully applied to the determination of drugs in various wastewaters to monitor environmental release of these substances. This analytical procedure is promising as it avoids the tedious extraction of large quantities of samples. Our findings are in accordance with other reported studies and corroborate the large differences to eliminate pharmaceuticals from urban wastewater in the treatment plant (Baker and Kasprzyk-Hordern 2011; Deblonde et al. 2011; Verlicchi et al. 2012; Pal et al. 2010). Based on these results, the amount of drugs and metabolites in the environment (i.e. TPWW outlet) represents large quantities as previously reported in the literature. Due to specific biological activities, it is imperative to improve their elimination in the treatment plant in order to avoid potential human and environmental exposure routes. Therefore, a highly specific, rapid on-line HPLC–MS/MS method is a very useful tool in order to assure the efficacy of the pharmaceutical wastewater treatment process.
References
Baker DR, Kasprzyk-Hordern B (2011) Multi-residue analysis of drugs of abuse in wastewater and surface water by solid-phase extraction and liquid chromatography-positive electrospray ionisation tandem mass spectrometry. J Chromato A 1218:1620–1631
Baranovska I, Kowalsi B (2012) A rapid UHPLC method for the simultaneous determination of drugs from different therapeutic groups in surface water and wastewater. Bull Environ Contam Toxicol 89:8–14
Deblonde T, Cossu-Leguille C, Hartemann P (2011) Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health 214:442–448
Escher BI, Baumgartner R, Koller M, Treyer K, Lienert J, McArdell CS (2011) Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res 45:75–92
Fatta-Kassinos D, Meric S, Nikolaou A (2011) Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research. Anal Bioanal Chem 399:251–275
Ge J, Cong J, Sun Y, Li G, Zhou Z, Qian C, Liu F (2010) Determination of endocrine disrupting chemicals in surface water and industrial wastewater from Beijing, China. Bull Enivron Contam Toxicol 84:401–405
Goullé JP, Saussereau E, Mahieu L, Cellier D, Spiroux J, Guerbet M (2012) Importance of anthropogenic metals in hospital and Urban Wastewater: it’s significance for the environment. Bull Environ Contam Toxicol 89:1220–1224
Lacroix C, Saussereau E, Bodin G, Goullé JP (2008) Quantification des opiacés, cocaïniques et amphétaminiques par chromatographie liquide haute performance/spectrométrie de masse tandem après préparation en ligne de l’échantillon. Ann Toxicol Anal 20:25–38
Le-Minh N, Khan SJ, Drewes JE, Stuetz RM (2010) Fate of antibiotics during municipal water recycling treatment processes. Water Res 44:4295–4323
Li J, Wang Z, Ma M, Peng X (2010) Analysis of environmental endocrine disrupting activities using recombinant yeast assay in wastewater treatment plant effluents. Bull Environ Contam Toxicol 84:29–35
Pal A, Gin KY, Lin AY, Reinhard M (2010) Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Sci Total Environ 408:6062–6069
Rowan NJ (2011) Defining established and emerging microbial risks in the aquatic environment: current knowledge, implications, and outlooks. Int J Microbiol 2011:462832
Tadeo JL, Sanchez-Brunette C, Albero B, Garcia-Valcarcel AI (2010) Determination of pesticide residues in sewage sludge: a review. J AOAC Int 93:1692–1702
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment—a review. Sci Total Environ 429:123–155
Acknowledgments
This research was supported by Grants from the Groupement Régional de Santé Publique de Haute-Normandie, Préfecture de Haute-Normandie, Rouen, France. This study was directed by the Union Régionale des Médecins Libéraux de Haute-Normandie, Rouen, France. The authors are most grateful to Richard Medeiros, Rouen University Hospital Medical Editor, for his valuable editing of the manuscript. The authors are also most grateful to Bernard Daumur, Rouen, University General Manager, for his support.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saussereau, E., Lacroix, C., Guerbet, M. et al. Determination of Levels of Current Drugs in Hospital and Urban Wastewater. Bull Environ Contam Toxicol 91, 171–176 (2013). https://doi.org/10.1007/s00128-013-1030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-013-1030-7