Abstract
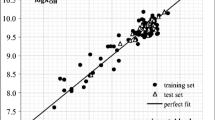
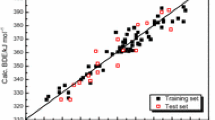
Eighteen phenol derivatives were optimized with density function theory (DFT) and comparative molecular similarity indices analysis (CoMSIA) respectively. Corresponding 2D and 3D descriptors were obtained to establish QSBR models. The biodegradation of them is mainly related to α and S ө according to the 2D QSBR, and influenced by the hydrophobicity and hydrogen bonding properties following the 3D QSBR. The 2D model performs better in stability and predictive ability than the 3D one. To some degrees, the two models verify and supplement each other. They can be used in predicting biodegradation of chlorinated, amine, nitro, nitroso and methyl phenol.



Similar content being viewed by others
References
Chen YS, Chen LX, Yang J, Zhuang YY, Dai SG (1997) A study on biodegradability of 32 aromatic compounds. Environ Chem 16:43–48
Chen YJ, Wang ZY, Mao L, Gao SX (2010) QSBR study on the biodegradation rate constant of chloro-phenol compounds. Chin J Struct Chem 29:895–899
Cui SH, Liu SS, Yang J, Wang XD, Wang LS (2006) Quantitative structure-activity relationship of estrogen activities of bisphenol A analogs. Sci China Phys Mech 51:287–292
Goi A, Trapido M, Tuhkanen T (2004) A study of toxicity, biodegradability, and some by-products of ozonised nitrophenols. Adv Environ Res 8:303–311
Gramatica P (2007) Principles of QSAR models validation: internal and external. QSAR Comb Sci 26:694–701
Hao RX, Li JB, Zhou YW, Cheng SY, Yi Z (2009) Structure–biodegradability relationship of nonylphenol isomers during biological wastewater treatment process. Chemosphere 75:987–994
Hsu YC, Yang HC, Chen JH (2005) The effects of preozonation on the biodegradability of mixed phenolic solution using a new gas-inducing reactor. Chemosphere 59:1279–1287
Jiang J, Chen JN, Yu HX, Zhang F, Zhang JF, Wang LS (2004) Quantitative structure activity relationship and toxicity mechanisms of chlorophenols on cells in vitro. Chin Sci Bull 49:562–566
Liu Y, Liu SS, Cui SH, Cai SX (2003) A novel quantitative structure-biodegradability relationship (QSBR) of substituted benzenes based on MHDV descriptor. J Chin Chem Soc 50:319–324
Mao L, Gao SX (2008) Reactions of estrogenic phenolic chemicals mediated by horseradish peroxidase: quantitative structure-activity relationships. Acta Sci Circumst 28:2562–2567
Pagga U (1997) Testing biodegradability with standardized methods. Chemosphere 35:2953–2972
Puzyn T, Suzuki N, Haranczyk M (2008) How do the partitioning properties of polyhalogenated POPs change when chlorine is replaced with bromine? Environ Sci Technol 42:5189–5195
Shi JQ, Liu HX, Sun L, Hou HF, Xu Y, Wang ZY (2011) Theoretical study on hydrophilicity and thermodynamic properties of polyfluorinated dibenzofurans. Chemosphere 84:296–304
Suarez-Ojeda ME, Fabregat A, Stuber F, Fortuny A, Carrera J, Font J (2007) Catalytic wet air oxidation of substituted phenols: temperature and pressure effect on the pollutant removal, the catalyst preservation and the biodegradability enhancement. Chem Eng J 132:105–115
Suarez-Ojeda ME, Carrera J, Metcalfe IS, Font J (2008) Wet air oxidation (WAO) as a precursor to biological treatment of substituted phenols: refractory nature of the WAO intermediates. Chem Eng J 144:205–212
Tabak HH, Rakesh G (1993) Prediction of biodegradation kinetics using a nonlinear group contribution method. Environ Toxicol Chem 12:251–260
Yang X, Liu HX, Hou HF, Flamm A, Zhang XS, Wang ZY (2010) Studies of thermodynamic properties and relative stability of a series of polyfluorinated dibenzo-p-dioxins by density functional theory. J Hazard Mater 181:969–974
Zhang SX, Golbraikh A, Tropsha A (2006) The development of quantitative structure-binding affinity relationship (QSBR) models based on novel geometrical chemical descriptors of the protein-ligand interfaces. J Med Chem 49:2713–2724
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Shi, J. 2D and 3D-QSBR Study on Biodegradation of Phenol Derivatives. Bull Environ Contam Toxicol 89, 316–321 (2012). https://doi.org/10.1007/s00128-012-0696-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0696-6