Abstract
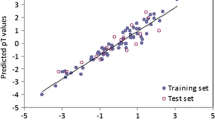
Quantitative structure–activity relationships (QSAR) is an alternative to experimental toxicity testing and recommended by environmental protection agencies. In this background, an accurate and reliable QSAR model of 18 phenols for their toxicity to Photobacterium phosphoreum was developed using mechanistically interpretable molecular structural descriptors. The QSAR model was developed by stepwise multiple linear regression and the reliability of the model was evaluated by internal and external validation. The cross-validated correlation coefficient (q 2) was 0.7021, indicating good predictive ability for the toxicity of these phenols. The QSAR model suggests that the toxicity of the studied compounds mainly depends on the logarithm of octanol/water partition coefficient, dipole moment and the most negative atomic charge.

Similar content being viewed by others
References
Aptula AO, Roberts DW, Cronin MT, Schultz TW (2005) Chemistry-toxicity relationships for the effects of di- and trihydroxybenzenes to Tetrahymena pyriformis. Chem Res Toxicol 18:844–854
Cronin M, Livingstone D (2004) Predicting chemical toxicity and fate. CRC Press, Boca Raton
Della GM, Monaco P, Pinto G, Pollio A, Previtera L, Temussi F (2001) Phytotoxicity of low-molecular-weight phenols from olive mill wastewaters. Bull Environ Contam Toxicol 67:352–359
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) AM1: A new general purpose quantum mechanical molecular model. J Am Chem Soc 107:3902–3909
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR (2003) Gaussian 03, Revision A.1. Gaussian, Inc., Pittsburgh
Habibi-Yangjeh A, Danandeh-Jenagharad M, Nooshyar M (2006) Application of artificial neural networks for predicting the aqueous acidity of various phenols using QSAR. J Mol Model 12:338–347
Jiang L, Lin ZF, Hu XL, Yin DQ (2010) Toxicity prediction of antibiotics on luminescent bacteria, photobacterium phosphoreum, based on their quantitative structure-activity relationship models. Bull Environ Contam Toxicol 85:550–555
Jin B, Liu C, Jin Q (2010) Quantitative structure-activity relationship for heterogeneous phenol compounds using zero point energy. Chin. J Struct Chem 29:1353–1361
Jing GH, Li XL, Zhou ZM (2010) Quantitative structure-activity relationship (QSAR) study of toxicity of substituted aromatic compounds to Photobacterium phosphoreum. Chin. J Struct Chem 29:1189–1196
McKim J, Schmieder P, Veith G (1985) Absorption dynamics of organic chemical transport across trout gills as related to octanol-water partition coefficient. Toxicol Appl Pharm 77:1–10
Niu JF, Yu G (2004) Molecular structural characteristics governing biocatalytic oxidation of PAHs with hemoglobin. Environ Toxicol Phar 18:39–45
Su LM, Yuan X, Mu CF, Yang JC, Zhao YH (2008) Evaluation and QSAR study of joint toxicity of substituted phenols and cadmium to Photobacterium phosphoreum. Chem Res Chin. U 24:281–284
Tang QY, Feng MG (2007) DPS data processing system: Experimental design, statistical analysis, and data mining. Science Press, Beijing
Verschueren K (2001) Handbook of environmental data on organic chemicals, 4th edn. Wiley, New York
Xu HY, Yu QS, Zou JW, Wang YH, Wang HQ, Chen XS (2006) A QSRR study on the relative retention time of halogenated methyl-phenyl ethers. Chin. J Struct Chem 25:811–817
Yu RL, Lin XY, Hu GR (2009) The joint toxicity of phenols to Photobacterium phosphoreum. J Huaqiao U 30:549–552
Zeng M, Lin ZF, Yin DQ, Zhang YL, Kong DY (2011) A K ow -based QSAR model for predicting toxicity of halogenated benzenes to all algae regardless of species. Bull Environ Contam Toxicol 86:565–570
Zhang HJ, Zhang JY, Zhu YM (2008) In vitro investigations for the QSAR mechanism of lymphocytes apoptosis induced by substituted aromatic toxicants. Fish Shellfish Immun 25:710–717
Acknowledgments
This work was supported by the National Natural Science Foundation of P. R. China (20737001, 20977046). All authors thank anonymous reviewers and editors for their valuable suggestions on revising and improving the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Wang, Z., Liu, H. et al. Quantitative Structure–Activity Relationship for Prediction of the Toxicity of Phenols on Photobacterium phosphoreum . Bull Environ Contam Toxicol 89, 27–31 (2012). https://doi.org/10.1007/s00128-012-0662-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0662-3