Abstract
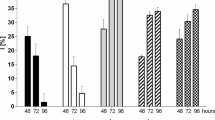
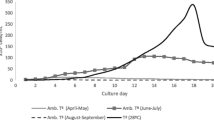
The fate of two trivalent chromium salts (nitrate and chloride) in ISO algal culture medium was followed over 72 h; i.e., the typical duration of algal toxicity tests. Fifty percent of the initial Cr spikes was lost from the solutions by 24 h, with losses up to 90% after 72 h. Monitoring of the temporal variability of Cr(III) concentrations in algal culture media appears necessary to better characterize the toxicity of trivalent chromium to algae.
Similar content being viewed by others
References
Baral A, Engelken R, Stephens W, Farris J, Hannigan R (2006) Evaluation of aquatic toxicities of chromium and chromium-containing effluents in reference to chromium electroplating industries. Arch Environ Contam Toxicol 50:496–502
Den Dooren de Jong LE (1965) Tolerance of Chlorella vulgaris for metallic and non-metallic ions. Antonie van Leeuwenhoek 31:301–313
Eary LE, Rai D (1987) Kinetics of chromium(III) oxidation to chromium(VI) by reaction with manganese dioxide. Environ Sci Technol 21:1187–1193
Greene JC, Miller WE, Debacon M, Long MA, Bartels CL (1988) Use of Selenastrum capricornutum to assess the toxicity potential of surface and groundwater contamination caused by chromium waste. Environ Toxicol Chem 7:35–39
INERIS (2005) Chrome et ses derivés. INERIS – DRC-01-05590-00DF253.doc. 80pp (in French)
ISO 8692 (2004) Water quality—Freshwater algal growth inhibition test with unicellular green algae. International Organization for Standardization, Geneva, Switzerland
IUCLID (1999) Chromium. International Uniform Chemical Information Database, European Commission, ISPRA
Koukal B, Dominik J, Vignati D, Arpagaus P, Santiago S, Ouddane B, Benaabidate L (2004) Assessment of water quality and toxicity of polluted rivers Fez and Sebou in the region of Fez (Morocco). Environ Pollut 131:163–172
Langmuir D (1997) Aqueous environmental geochemistry. Prentice-Hall, New Jersey, 600 pp (Figure 2.2; p 54)
Lin CJ (2002) The chemical transformations of chromium in natural waters—A model study. Water Air Soil Pollut 139:137–158
Meisch HU, Schmitt-Beckmann I (1979) Influence of tri and hexavalent chromium on two Chlorella strains. Zeitschrift für Pflanzenphysiologie Bd 94:231–239
Munn SJ, Allanou R, Aschberger K, Berthault F, Cosgrove O, Luotamo M, Pakalin S, Paya-Perez A, Pellegrini G, Schwarz-Schulz B, Vegro S (eds) (2005) Chromium trioxide, sodium chromate, sodium dichromate, ammonium dichromate, potassium dichromate, EUR 21508 EN. European Union Risk Assessment Report, vol 53. Luxembourg, Office for Official Publications of the European Communities
Pawlisz AV, Kent RA, Schneider UA, Jefferson C (1997) Canadian water quality guidelines for chromium. Environ Toxicol Water Qual 12:123–183
Rai D, Eary LE, Zachara JM (1989) Environmental chemistry of chromium. Sci Total Environ 86:15–23
Rai D, Moore DA, Hess NJ, Rao L, Clark SB (2004) Chromium(III) hydroxide solubility in the aqueous Na+–OH–H2PO4–HPO42–H2O system: a thermodynamic model. J Solut Chem 33:1213–1242
Rozan TM, Lassman ME, Ridge DP, Luther III GW (2000) Evidence for iron, copper and zinc complexation as multinuclear sulphide clusters in oxic river waters. Nature 406:879–882
Sass BM, Rai D (1987) Solubility of amorphous chromium(III)-iron(III) hydroxide solid solutions. Inorg Chem 26:2228–2282
Stoecker B (2004) Chromium. In: Merian E, Anke M, Ihnat M, Stoeppler M (eds) Elements and their compounds in the environment, 2nd edn, vol 2—Metals and their compounds. Wiley-VCH, Weinheim, Germany, pp 709–729
Town RM, Filella M (2002) Implications of natural organic matter binding heterogeneity on understanding lead (II) complexation in aquatic systems. Sci Total Environ 300:143–154
Turbak SC, Olson SB, McFeters GA (1986) Comparison of algal assay systems for detecting waterborne herbicides and metals. Water Res 20:91–96
Walsh AR, O’Halloran J (1997) The accumulation of chromium by mussels Mytilus edulis (L.) as a function of valency, solubility and ligation. Mar Environ Res 43:41–53
Acknowledgements
This research was partially supported by the Swiss National Science Foundation (grant 200020-101844). Dr. Pierre-Yves Favarger (Institut F.-A. Forel) helped with ICP-MS analysis and Sandra Levai (University of Geneva) with the search of primary literature sources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vignati, D.A.L., Beye, M.L., Dominik, J. et al. Temporal Decrease of Trivalent Chromium Concentration in a Standardized Algal Culture Medium: Experimental Results and Implications for Toxicity Evaluation. Bull Environ Contam Toxicol 80, 305–310 (2008). https://doi.org/10.1007/s00128-008-9379-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-008-9379-8