Abstract
Aims/hypothesis
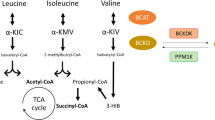
An increasing body of evidence has shown that the catabolism of branched-chain amino acids (BCAAs; leucine, isoleucine and valine) is impaired in obese animals and humans, contributing to the development of insulin resistance and type 2 diabetes. Promoting BCAA catabolism benefits glycaemic control. It remains unclear whether BCAA catabolism plays a role in the therapeutic efficacy of currently used glucose-lowering drugs such as metformin.
Methods
Mice were treated with vehicle or metformin (250 mg/kg per day) for more than 4 weeks to investigate the effects of metformin in vivo. In vitro, primary mouse hepatocytes and HepG2 cells were treated with 2 mmol/l metformin. The therapeutic efficacy of metformin in the treatment of type 2 diabetes was assessed in genetically obese (ob/ob) mice and high-fat-diet-induced obese (DIO) mice. Enhancing BCAA catabolism was achieved with a pharmacological agent, 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2). The ob/ob mice were treated with a low-BCAA diet or intermittent protein restriction (IPR) to reduce BCAA nutritional intake.
Results
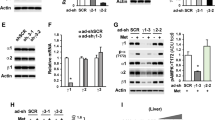
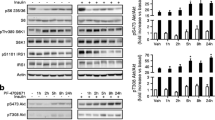
Metformin unexpectedly inhibited the catabolism of BCAAs in obese mice, resulting in an elevation of BCAA abundance. AMP-activated protein kinase (AMPK) mediated the impact of metformin on BCAA catabolism in hepatocytes. Importantly, enhancing BCAA catabolism via a pharmacological agent BT2 significantly potentiated the glucose-lowering effect of metformin while decreasing circulating BCAA levels in ob/ob and DIO mice. Similar outcomes were achieved by a nutritional approach of reducing BCAA intake. IPR also effectively reduced the circulating BCAA abundance and enhanced metformin’s glucose-lowering effect in ob/ob mice. BT2 and IPR treatments reduced the expression of fructose-1,6-bisphosphatase 1, a rate-limiting enzyme in gluconeogenesis, in the kidney but not liver, indicating the involvement of renal gluconeogenesis.
Conclusions/interpretation
Metformin self-limits its therapeutic efficacy in the treatment of type 2 diabetes by triggering the suppression of BCAA catabolism. Enhancing BCAA catabolism pharmacologically or reducing BCAA intake nutritionally potentiates the glucose-lowering effect of metformin. These data highlight the nutritional impact of protein on metformin’s therapeutic efficacy and provide new strategies targeting BCAA metabolism to improve metformin’s effects on the clinical outcome in diabetes.
Graphical Abstract








Similar content being viewed by others
Abbreviations
- AICAR:
-
5-Aminoinidazole 4-carboxamide 1-β-d-ribofuranoside
- AMPK:
-
AMP-activated protein kinase
- BCAA:
-
Branched-chain amino acid
- BCAT:
-
Branched-chain aminotransferase
- BCKA:
-
Branched-chain keto acid
- BCKD:
-
BCKA dehydrogenase
- BCKDK:
-
BCKD kinase
- BCKDK-LKO:
-
Liver-specific Bckdk-knockout
- BT2:
-
3,6-Dichlorobenzo[b]thiophene-2-carboxylic acid
- DIO:
-
High-fat-diet-induced obese
- FBP1:
-
Fructose-1,6-bisphosphatase 1
- HFD:
-
High-fat diet
- IPR:
-
Intermittent protein restriction
- L-BCAA:
-
Low-BCAA
- LPD:
-
Low-protein diet
- NPD:
-
Normal protein diet
- PP2Cm:
-
Protein phosphatase 2Cm
- RT-qPCR:
-
Quantitative RT-PCR
References
Foretz M, Guigas B, Viollet B (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol 15(10):569–589. https://doi.org/10.1038/s41574-019-0242-2
Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA (2016) Metformin as a tool to target aging. Cell Metab 23(6):1060–1065. https://doi.org/10.1016/j.cmet.2016.05.011
Morales DR, Morris AD (2015) Metformin in cancer treatment and prevention. Annu Rev Med 66:17–29. https://doi.org/10.1146/annurev-med-062613-093128
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia 60(9):1577–1585. https://doi.org/10.1007/s00125-017-4342-z
El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275(1):223–228. https://doi.org/10.1074/jbc.275.1.223
Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348(Pt 3):607–614. https://doi.org/10.1042/bj3480607
Hawley SA, Ross FA, Chevtzoff C et al (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11(6):554–565. https://doi.org/10.1016/j.cmet.2010.04.001
Ross FA, MacKintosh C, Hardie DG (2016) AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J 283(16):2987–3001. https://doi.org/10.1111/febs.13698
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262. https://doi.org/10.1038/nrm3311
Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494(7436):256–260. https://doi.org/10.1038/nature11808
Hunter RW, Hughey CC, Lantier L et al (2018) Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat Med 24(9):1395–1406. https://doi.org/10.1038/s41591-018-0159-7
Ma T, Tian X, Zhang B et al (2022) Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603(7899):159–165. https://doi.org/10.1038/s41586-022-04431-8
Steinberg GR, Hardie DG (2023) New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol 24(4):255–272. https://doi.org/10.1038/s41580-022-00547-x
Blair MC, Neinast MD, Arany Z (2021) Whole-body metabolic fate of branched-chain amino acids. Biochem J 478(4):765–776. https://doi.org/10.1042/BCJ20200686
White PJ, McGarrah RW, Herman MA, Bain JR, Shah SH, Newgard CB (2021) Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol Metab 52:101261. https://doi.org/10.1016/j.molmet.2021.101261
White PJ, Newgard CB (2019) Branched-chain amino acids in disease. Science 363(6427):582–583. https://doi.org/10.1126/science.aav0558
Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10(12):723–736. https://doi.org/10.1038/nrendo.2014.171
Harper AE, Miller RH, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4:409–454. https://doi.org/10.1146/annurev.nu.04.070184.002205
Neinast MD, Jang C, Hui S et al (2019) Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab 29(2):417–429. https://doi.org/10.1016/j.cmet.2018.10.013. (e4)
Popov KM, Zhao Y, Shimomura Y, Kuntz MJ, Harris RA (1992) Branched-chain alpha-ketoacid dehydrogenase kinase. Molecular cloning, expression, and sequence similarity with histidine protein kinases. J Biol Chem 267(19):13127–13130
Zhou M, Shao J, Wu CY et al (2019) Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 68(9):1730–1746. https://doi.org/10.2337/db18-0927
White PJ, McGarrah RW, Grimsrud PA et al (2018) The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab 27(6):1281–1293. https://doi.org/10.1016/j.cmet.2018.04.015. (e7)
Vanweert F, Neinast M, Tapia EE et al (2022) A randomized placebo-controlled clinical trial for pharmacological activation of BCAA catabolism in patients with type 2 diabetes. Nat Commun 13(1):3508. https://doi.org/10.1038/s41467-022-31249-9
Shao J, Liu Y, Zhang X et al (2022) BCAA catabolism drives adipogenesis via an intermediate metabolite and promotes subcutaneous adipose tissue expansion during obesity [preprint]. bioRxiv. https://doi.org/10.1101/2022.08.18.504380
Qin X, Zhang J, Wang B et al (2021) Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 17(12):4266–4285. https://doi.org/10.1080/15548627.2021.1911016
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89(3):1025–1078. https://doi.org/10.1152/physrev.00011.2008
Burkewitz K, Zhang Y, Mair WB (2014) AMPK at the nexus of energetics and aging. Cell Metab 20(1):10–25. https://doi.org/10.1016/j.cmet.2014.03.002
Liu X, Chhipa RR, Nakano I, Dasgupta B (2014) The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther 13(3):596–605. https://doi.org/10.1158/1535-7163.MCT-13-0579
Tso SC, Qi X, Gui WJ et al (2013) Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain α-ketoacid dehydrogenase kinase. Proc Natl Acad Sci 110(24):9728–9733. https://doi.org/10.1073/pnas.1303220110
Sun H, Olson KC, Gao C et al (2016) Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133(21):2038–2049. https://doi.org/10.1161/CIRCULATIONAHA.115.020226
Tso SC, Gui WJ, Wu CY et al (2014) Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain alpha-ketoacid dehydrogenase kinase. J Biol Chem 289(30):20583–20593. https://doi.org/10.1074/jbc.M114.569251
Yoneshiro T, Wang Q, Tajima K et al (2019) BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572(7771):614–619. https://doi.org/10.1038/s41586-019-1503-x
Neinast M, Murashige D, Arany Z (2019) Branched chain amino acids. Annu Rev Physiol 81:139–164. https://doi.org/10.1146/annurev-physiol-020518-114455
Levine ME, Suarez JA, Brandhorst S et al (2014) Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19(3):407–417. https://doi.org/10.1016/j.cmet.2014.02.006
Mirzaei H, Raynes R, Longo VD (2016) The conserved role of protein restriction in aging and disease. Curr Opin Clin Nutr Metab Care 19(1):74–79. https://doi.org/10.1097/MCO.0000000000000239
Cheng CW, Villani V, Buono R et al (2017) Fasting-mimicking diet promotes NGN3-driven beta-cell regeneration to reverse diabetes. Cell 168(5):775–788. https://doi.org/10.1016/j.cell.2017.01.040. (e12)
Hoffer LJ, Taveroff A, Schiffrin A (1997) Metabolic adaptation to protein restriction in insulin-dependent diabetes mellitus. Am J Physiol 272(1 Pt 1):E59-67. https://doi.org/10.1152/ajpendo.1997.272.1.E59
Wei S, Li C, Luo X et al (2022) Intermittent protein restriction protects islet beta cells and improves glucose homeostasis in diabetic mice. Sci Bull (Beijing) 67(7):733–747. https://doi.org/10.1016/j.scib.2021.12.024
Legouis D, Faivre A, Cippa PE, de Seigneux S (2022) Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrol Dial Transplant 37(8):1417–1425. https://doi.org/10.1093/ndt/gfaa302
Zemdegs J, Martin H, Pintana H et al (2019) Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci 39(30):5935–5948. https://doi.org/10.1523/JNEUROSCI.2904-18.2019
Lian K, Du C, Liu Y et al (2015) Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 64(1):49–59. https://doi.org/10.2337/db14-0312
Fontana L, Cummings NE, Arriola Apelo SI et al (2016) Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 16(2):520–530. https://doi.org/10.1016/j.celrep.2016.05.092
Cummings NE, Williams EM, Kasza I et al (2018) Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 596(4):623–645. https://doi.org/10.1113/JP275075
White PJ, Lapworth AL, An J et al (2016) Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab 5(7):538–551. https://doi.org/10.1016/j.molmet.2016.04.006
Gerich JE, Meyer C, Woerle HJ, Stumvoll M (2001) Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24(2):382–391. https://doi.org/10.2337/diacare.24.2.382
Schoolwerth AC, Smith BC, Culpepper RM (1988) Renal gluconeogenesis. Miner Electrolyte Metab 14(6):347–361
Papakonstantinou E, Oikonomou C, Nychas G, Dimitriadis GD (2022) Effects of diet, lifestyle, chrononutrition and alternative dietary interventions on postprandial glycemia and insulin resistance. Nutrients 14(4):823. https://doi.org/10.3390/nu14040823
Dahabiyeh LA, Mujammami M, Arafat T, Benabdelkamel H, Alfadda AA, Abdel Rahman AM (2021) A metabolic pattern in healthy subjects given a single dose of metformin: a metabolomics approach. Front Pharmacol 12:705932. https://doi.org/10.3389/fphar.2021.705932
Walford GA, Davis J, Warner AS et al (2013) Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism 62(12):1772–1778. https://doi.org/10.1016/j.metabol.2013.07.003
Hermann LS (1990) Biguanides and sulfonylureas as combination therapy in NIDDM. Diabetes Care 13(Suppl 3):37–41. https://doi.org/10.2337/diacare.13.3.37
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355(23):2427–2443. https://doi.org/10.1056/NEJMoa066224
Cook MN, Girman CJ, Stein PP, Alexander CM (2007) Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with type 2 diabetes in UK primary care. Diabet Med 24(4):350–358. https://doi.org/10.1111/j.1464-5491.2007.02078.x
Bailey CJ, Turner RC (1996) Metformin. N Engl J Med 334(9):574–579. https://doi.org/10.1056/NEJM199602293340906
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Data availability
Data generated in this study are available from the corresponding author upon request.
Funding
This work was supported by the National Key Research and Development Program of China (2019YFA0802503), the National Natural Science Foundation of China (92057107, 81570717, 31900819, 32200965, 82270856), the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01) and the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-032A).
Authors’ relationships and activities
HS participated in an advisory board for Ramino Bio Ltd. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
HS and WN designed the study. XZhao, XZhang, JP and YL performed the experiments, contributed to data analysis and drafted the manuscript. HS and WN reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript. HS and WN are responsible for the integrity of the work as a whole.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Zhang, X., Pei, J. et al. Targeting BCAA metabolism to potentiate metformin’s therapeutic efficacy in the treatment of diabetes in mice. Diabetologia 66, 2139–2153 (2023). https://doi.org/10.1007/s00125-023-05985-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05985-6