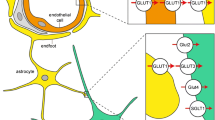
Abstract
Aims/hypothesis
We have previously shown that diabetes causes pericyte dysfunction, leading to loss of vascular integrity and vascular cognitive impairment and dementia (VCID). Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1 RAs), used in managing type 2 diabetes mellitus, improve the cognitive function of diabetic individuals beyond glycaemic control, yet the mechanism is not fully understood. In the present study, we hypothesise that GLP-1 RAs improve VCID by preventing diabetes-induced pericyte dysfunction.
Methods
Mice with streptozotocin-induced diabetes and non-diabetic control mice received either saline (NaCl 154 mmol/l) or exendin-4, a GLP-1 RA, through an osmotic pump over 28 days. Vascular integrity was assessed by measuring cerebrovascular neovascularisation indices (vascular density, tortuosity and branching density). Cognitive function was evaluated with Barnes maze and Morris water maze. Human brain microvascular pericytes (HBMPCs), were grown in high glucose (25 mmol/l) and sodium palmitate (200 μmol/l) to mimic diabetic conditions. HBMPCs were treated with/without exendin-4 and assessed for nitrative and oxidative stress, and angiogenic and blood–brain barrier functions.
Results
Diabetic mice treated with exendin-4 showed a significant reduction in all cerebral pathological neovascularisation indices and an improved blood–brain barrier (p<0.05). The vascular protective effects were accompanied by significant improvement in the learning and memory functions of diabetic mice compared with control mice (p<0.05). Our results showed that HBMPCs expressed the GLP-1 receptor. Diabetes increased GLP-1 receptor expression and receptor nitration in HBMPCs. Stimulation of HBMPCs with exendin-4 under diabetic conditions decreased diabetes-induced vascular inflammation and oxidative stress, and restored pericyte function (p<0.05).
Conclusions/interpretation
This study provides novel evidence that brain pericytes express the GLP-1 receptor, which is nitrated under diabetic conditions. GLP-1 receptor activation improves brain pericyte function resulting in restoration of vascular integrity and BBB functions in diabetes. Furthermore, the GLP-1 RA exendin-4 alleviates diabetes-induced cognitive impairment in mice. Restoration of pericyte function in diabetes represents a novel therapeutic target for diabetes-induced cerebrovascular microangiopathy and VCID.
Graphical abstract

Similar content being viewed by others

Introduction
Diabetes mellitus is a rising health problem worldwide, causing multiple cerebrovascular complications, including stroke, retinopathy and vascular cognitive impairments and dementia (VCID) [1,2,3]. Poor glycaemic control in diabetic individuals is correlated with worsening cognitive outcomes [3, 4]. With no known therapeutic options, VCID treatments are limited only to glycaemic control.
Glucagon-like peptide-1 (GLP-1) is an incretin peptide hormone secreted by the L cells of the gastrointestinal tract. GLP-1 stimulates glucose-dependent insulin secretion and beta cell proliferation [5]. GLP-1 receptors (GLP-1Rs) are G-protein-coupled receptors located on cells in the heart, brain, pancreas and stomach [6]. GLP-1R agonists (GLP-1RAs) decrease glucagon production, improve insulin secretion, increase satiety and reduce food intake [6]. According to the ADA guidelines, GLP-1RAs are among the first-line treatments for type 2 diabetes mellitus because of their glycaemic control benefits and cardiovascular protective capabilities [7, 8]. GLP-1RAs may have neuroprotective effects in individuals with Alzheimer’s disease and dementia beyond glycaemic control [9]. However, the mechanisms underlying the neuroprotective effects of GLP-1RAs are not fully known and are still under investigation [10].
Pericytes are centred among cells that compose the neurovascular unit. Structurally, pericytes are the cells that wrap the endothelial cells and share the basement membrane with them [11, 12]. Pericytes are responsible for stabilising the capillaries, controlling blood flow and permeability, and coordinating angiogenesis [13, 14]. Diabetes increases pericytes loss; this compromises vascular integrity and causes cerebrovascular pathological neovascularisation [14,15,16].
In the present study, we explored the expression of the GLP-1R in brain pericytes under normal and diabetic conditions. Furthermore, we examined the impact of a GLP-1RA on pericyte function in diabetes. The current project tests the hypothesis that diabetes induces GLP-1R nitration, leading to pericyte dysfunction and loss of cerebrovascular integrity and VCID.
Methods
Animals
C57BL/6J mice, Pdgfr-β-CreERT2 transgenic mice [B6.Cg-Tg(Pdgfrb-cre/ERT2)6096Rha/J, The Jackson Laboratory, USA; https://www.jax.org/strain/029684] and Ai14 mice [B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, The Jackson Laboratory, USA; https://www.jax.org/strain/007914] were purchased from Jax lab (Ellsworth, Maine, USA) and successfully inbred in the Mercer animal facility. All animal procedures were approved by the Mercer University Institution Animal Care and Use Committee (IACUC). Mercer University IACUC is accredited by the American Association for Accreditation of Laboratory Animal Care. Mice were fed standard rat chow and tap water ad libitum and were maintained under a 12 h light–dark cycle.
PC-Tomato mice are Cre/loxP transgenic mice generated by in-house breeding of Pdgfr-β-CreERT2 transgenic mice with Ai14 mice to express red fluorescence protein in pericytes. All mouse strains were genotyped according to Jax lab protocol (for primers, see electronic supplementary material [ESM] Table 1). PC-Tomato mice were identified by PCR to have both the Pdgfr-β-CreERT2 and dtTomato genes. A single i.p. injection of tamoxifen (100 mg/kg body weight) was administered to PC-Tomato mice 2 weeks before sacrifice to induce the Cre-loxP system.
Induction of diabetes and treatment
At 8 weeks old, male C57BL/6J and PC-Tomato mice were fasted overnight and assigned to receive one i.p. injection of vehicle or a low dose of streptozotocin (STZ) (35 mg/kg body weight; Alfa Aesar, Tewksbury, MA, USA) [15]. Mice with blood glucose levels over 13.9 mmol/l (250 mg/dl) were identified as diabetic and were kept on a high-fat diet (HFD) (45% energy from fat; Research Diets, New Brunswick, NJ, USA) for 8 weeks. Control mice were fed standard rat chow (10% energy from fat, Research Diets). Body weight and blood glucose were monitored weekly (ESM Table 2).
Four weeks after induction of diabetes, mice were randomly assigned to receive vehicle (saline, 154 mmol/l NaCl) or exendin-4 treatment. An osmotic pump, implanted subcutaneously (MODEL 1004; 0.11 μl/h, 28-day release; Azlet, Cupertino, CA, USA) was used to release either vehicle or exendin-4 (35 μg kg−1 day−1; Tochris Bioscience, Bristol, UK) over the course of 4 weeks. The dose was selected according to previously published data [17, 18]. Animal behaviour testing was performed 8 days before sacrifice.
Assessment of cognitive impairment and dementia
Barnes maze
Barnes maze from Stoelting (Wood Dale, IL, USA), consisting of 20 evenly spaced holes around the table’s perimeter, was used to assess spatial reference learning and memory. Nineteen of the holes are ‘false escapes’ where the mice cannot effectively hide. One hole contains the escape box (goal zone), which leads to a dark recess below the table, serving as an escape from the stressor. One week before sacrifice, mice were trained three times daily for a duration of 3 days. On testing day, the goal box was replaced with a false escape. ANYmaze software (version 6.1; Stoelting) was used to measure the time spent and the number of entries into the goal quadrant.
Morris water maze
Morris water maze (Stoelting) was used to assess learning and memory functions. The Morris water maze consists of a water pool that contains a platform. One week before sacrifice, mice were trained to find the platform using visual cues that assist in orientation three times each day for three days. On testing day, the platform was removed and movement of the mice was tracked using ANYmaze software to measure the time spent in, and number of entries to, the goal zone.
Vascular integrity and pathological neovascularisation assessment
To examine the effect of GLP-1RAs on pathological neovascularisation, mouse brains were collected and fixed in 2% paraformaldehyde followed by dehydration in 30% sucrose solution. Brains were sectioned at a thickness of 30–45 μm using Leica CM3050 S Sectioner (Leica Biosystems, Buffalo Grove, IL, USA) [15, 16, 19]. Brain sections were stained with Isolectin B4 DyLight 594 (Vector Laboratories, USA) overnight. Z-stack images were taken using a Nikon Eclipse Ti-E Inverted Confocal Microscope (Nikon Instruments, Japan). Reconstructed 3D images were processed to analyse neovascularisation indices using FIJI software, an image processing and analysis version of the ImageJ software (https://hpc.nih.gov/apps/Fiji.html), blindly. Vascular density refers to the density of stained vasculature from the merged planes over the total number of planes in the section. Branching density was calculated as the ratio between the numbers of branches over the longest shortest branch path. For vessel tortuosity, tortuosity index was calculated as the ratio between branch length and Euclidian distance of the branch.
Cell culture
Two different lots of human brain pericytes were purchased from Angio-Proteomie (Boston, MA, USA) and grown using a complete medium (Pericyte Growth Medium [Super Rich Formulation, containing FBS and growth factors], catalogue no. CAP09B; Boston, MA, USA). All experiments were performed in triplicate using cells between passages 4 and 6. Cells were switched to a serum-free medium before performing cell migration assays or treatment applications. High glucose (20 mmol/l) and sodium palmitate (200 μmol/l) were added to mimic the diabetic conditions. Equimolar l-glucose was used as an osmotic control. Exendin-4 treatment was used at concentration of 100 nmol/l and epicatechin, a selective nitration inhibitor, was used at concentration of 10 μmol/l.
Mouse pericyte isolation
Primary brain microvascular pericytes were isolated from wild-type mice by an immunomagnetic method of separation using Dynabeads (Invitrogen, Carlsbad, CA, USA) as described previously [20]. The identity of primary brain pericytes was confirmed using immunohistochemical staining with pericyte markers (ESM Fig. 1).
PCR
RNA was isolated from cellular or brain tissue using Triazol (Invitrogen) and quantified using Thermo Scientific NanoDrop 2000C Spectrophotometer (Thermo Scientific, USA). Quantitative PCR was conducted using QuantStudio 3 Real-Time PCR System (Applied Biosystems, Thermo Scientific). All primers sequences used are shown in ESM Table 1.
Migration assay
Pericytes were exposed to diabetic conditions (200 μmol/l of sodium palmitate and 25 mmol/l d-glucose) for 3 days and then switched to reduced-serum medium with the same glucose level for two more days. After a total of 5 days in hyperglycaemic conditions, a green cell tracker (catalogue no. E34251; Invitrogen) was added for 1 h for easy cell visualisation. Cells were scratched and imaged for zero time after 24 h. Results are presented as relative per cent migration ± SEM.
3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide viability assay
For the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) viability assay, pericytes were seeded in a 96-well plate and switched to normal glucose (5 mmol/l) or high glucose media with/without exendin-4 (100 nmol/l) for 48 h. MTT Cell Proliferation Assay Kit (item no. 10009365; Cayman Chemical, Ann Arbor, MI, USA) was used to determine cell viability following the manufacturers’ instructions.
Oxidative stress assessment
Pericytes were grown to confluency in complete medium in a 96-well plate. Cells were switched to normal glucose or high glucose media with/without exendin-4 (100 nmol/l) for 48 h. Oxidative stress was assessed using ROS Detection Cell-Based Assay Kit (DHE) (item no. 601290; Cayman Chemical, Ann Arbor, MI, USA).
Glutathione assay
Glutathione (GSH GSSG) Assay Kit (NWLSS, Northwest Life Science Specialties, USA) was used to assess the oxidised and reduced glutathione according to the manufacturer’s protocol. Serum samples from control, diabetic or exendin-4-treated diabetic mice were reacted with 2,2′-dithiobis(5-nitropyridine) (DTNP) in the presence and absence of 4-vinylpuridine (4-VP), a glutathione chelator. Samples were then reduced using glutathione reductase and NADPH. Colour absorbance was measured using a BioTek Synergy HT (BioTek U.S., Winooski, VT, USA) in the program Gen5 (BioTek U.S.) at 413 nm. The reduced glutathione was calculated by subtracting oxidised glutathione (samples with 4-VP) from the total glutathione (samples without 4-VP).
GLP-1R silencing
GLP-1Rs were silenced using siRNA technology. Pericytes were transfected with either scrambled or Glp1r siRNA (Santa Cruz, Santa Cruz, CA, USA) using the Amaxa nucleofector kit (Lonza, Koeln, Germany) according to the manufacturer’s protocol. Experiments were performed in triplicates within 72 hours after transfection. Transfection efficacy was confirmed using Green Fluorescent Protein expression and immunoblotting (EMS Fig. 2).
Immunohistochemical staining
Pericytes were seeded in 4-well chamber slides at 50% confluency, incubated overnight with GLP-1 receptor primary antibody (1:100; Novus Biologicals, Centennial, CO, USA). The following day, the cells were incubated with the secondary antibody goat anti-mouse tagged with Alexa Fluor 488 (1:1000, catalogue no. A28175; Invitrogen). DAPI mounting media was added and cells were imaged with a Nikon (Japan) Eclipse Ti confocal/fluorescent microscope.
Brain sections were washed, then blocked using 0.01% horse serum, and incubated overnight with GLP-1R antibody (1:150, Novus Biologicals) or occludin antibody (1:150, catalogue no. 13409-1-AP; Proteintech, Rosemont, IL, USA). Sections were then reacted in the dark with a green fluorescent secondary antibody (1:1000), and imaged using a Nikon Eclipse Ti-E Inverted Microscope (Nikon Instruments, Melville, NY, USA) in the NiS Elements AR programme. Image J was used for quantification.
Western blot and slot blot analysis
Brain samples and pericytes were homogenised in RIPA buffer (catalogue no. 3P 20188; Millipore, Billerica, MA, USA). Using electrophoresis, samples were separated on a 4–15% gradient sodium dodecyl sulfate–polyacrylamide gel (Bio-Rad, CA, USA). Gels were then transferred to a nitrocellulose membrane. For slot blot, equal protein loading was applied on membrane directly with vacuum using slot blot apparatus (Bio-Rad). Membranes were then incubated with the primary antibodies (1:500), followed by the corresponding horseradish peroxidase-conjugated secondary antibody (1:5000). The membrane was treated with a Western chemiluminescent HRP Substrate (Millipore), and imaged using an Azure Biosystems c600 (Azure Biosystems, Dublin, CA, USA). Band intensity was quantified using densitometry Image J software (see ESM Table 3 for a complete list of the antibodies used).
Immunoprecipitation
Pericytes lysate was immunoprecipitated with a GLP-1R antibody followed by protein A/G agarose beads. Samples were separated on a sodium dodecyl sulfate–polyacrylamide gel, then transferred to nitrocellulose membranes and incubated with nitrotyrosine polyclonal antibody. GLP-1R polyclonal antibody (Novus) was used as a loading control.
Statistical analysis
Statistical significance for all analyses was assessed at an α level of 0.05 using GraphPad Prism version 8 or later (GraphPad, USA). A Tukey–Kramer adjustment for multiple comparisons was used for all post hoc mean comparisons. One-way ANOVA was used to compare the means of groups in all figures. Results were reported as the mean percentage of control ± SEM.
Results
Diabetes increases GLP-1R expression in brain pericytes
The GLP-1R is expressed in brain tissue, mainly in neuronal, glial, vascular smooth muscle and endothelial cells [21,22,23]. We used multiple approaches to detect whether GLP-1Rs are expressed in human brain microvascular pericyte cells (HBMPCs) and to examine the effect of diabetes on GLP-1R expression.
HBMPCs were grown in normal glucose or high glucose/palmitate concentrations to mimic diabetic conditions. Immunohistochemical studies showed that HBMPCs express GLP-1Rs, and that the expression was significantly increased under diabetic conditions (Fig. 1a). RT-PCR and western blot analysis confirmed that HBMPCs expressed GLP-1Rs, with a significant increase in expression (fourfold and 1.9-fold, respectively) under diabetic conditions when compared with control conditions (Fig. 1b,c).
Diabetes increases GLP-1R expression in brain pericytes. (a–c) HBMPCs were grown to confluency in complete media then switched to normal glucose (control, 5 mmol/l) or to high glucose (25 mmol/l) and sodium palmitate (200 μmol/l) to mimic diabetic conditions for 5 days. Representative immunohistochemical image of GLP-1Rs in pericytes (stained green) and quantification (a) showing that HBMPCs express GLP-1Rs and that diabetic conditions significantly increase this expression (*p<0.05 vs control conditions, n = 3). RT-PCR analysis (b) showed that pericytes express Glp1r mRNA and that diabetic conditions significantly increase Glp1r mRNA levels (*p<0.05 vs control conditions, n = 3). Western blot analysis (c) showed that pericytes express GLP-1Rs and that diabetic conditions significantly increase GLP-1R expression (*p<0.05 vs control conditions, n = 4). (d) In parallel, the expression of GLP-1R in diabetic mice was examined. PC-Tomato mice were injected with STZ (35 mg/kg body weight) for 8 weeks to induce diabetes. Brains were isolated and stained for GLP-1R. Representative images and quantification for immunohistochemical staining of GLP-1Rs (green) colocalised on pericytes (red), showing that pericytes express GLP-1R and that the expression is increased in brains of diabetic mice (*p<0.05 vs non-diabetic mice, n = 4). (e, f) Mouse brain pericytes were isolated and assessed for GLP-1R expression using RT-PCR and western blot. The pericytes were grown to confluency in complete media then switched to normal glucose (control) or high glucose (25 mmol/l) and sodium palmitate (200 μmol/l) to mimic diabetic conditions. RT-PCR analysis (e) showed that mouse pericytes express Glp1r mRNA and that diabetic conditions significantly increase Glp1r mRNA levels (*p<0.05 vs control conditions, n = 3). Western blot analysis (f) showed that the mouse brain pericytes express GLP-1Rs and that diabetic conditions significantly increase the expression (*p<0.05 vs control conditions, n = 4). (g, h) Whole brains were homogenised, and Glp1r mRNA levels were assessed. RT-PCR analysis (g) showed that brain Glp1r mRNA expression significantly increases under diabetic conditions (*p<0.05 vs control conditions, n = 3 or 4) and western blot analysis (h) showed that brain GLP-1R expression was also significantly increased in diabetes (*p<0.05 vs non-diabetic mice, n = 3 or 4). Scale bar, 50 μm. Data are presented as mean percentage of control ± SEM. HG, high glucose; NG, normal glucose; Pal, sodium palmitate
The expression of GLP-1R in brain pericytes was investigated in vivo using immunohistochemical studies and immunoblotting. Immunohistochemical colocalisation studies for brain pericytes are challenging because pericytes share many markers with neuronal and vascular smooth muscle cells, such as nerve/glial antigen 2 (NG-2) and platelet-derived growth factor receptor β (PDGFR-β) markers. To overcome this challenge, we used a well-established transgenic mouse model expressing red fluorescence in pericytes [24]. In PC-Tomato mice (tamoxifen-induced Cre-Lox P transgenic mice), pericytes and vascular smooth muscle cells express a red fluorescent protein. PC-Tomato made the pericyte colocalisation studies more accurate and reliable since we target small brain capillaries with no vascular smooth muscles. Our results showed the colocalisation of GLP-1R with the pericytes (Fig. 1d).
Next, we isolated primary brain pericytes from wild-type mice and examined the expression of GLP-1R. RT-PCR and western blot analysis confirmed that mouse pericytes expressed GLP-1Rs, with a significant increase in the expression (fourfold and 2.2-fold, respectively) under diabetic conditions when compared with control conditions (Fig. 1e,f). We examined the effect of diabetes on GLP-1R expression in in-vivo settings. Diabetes was induced in PC-Tomato mice (HFD and low-dose STZ for 8 weeks). Immunohistochemical studies showed that diabetes increased the GLP-1R expression in brain pericytes. Moreover, our results showed a significant increase in Glp1r mRNA levels and GLP-1R protein expression in whole-brain homogenate using RT-PCR and immunoblotting, respectively (Fig. 1g,h).
GLP-1RA prevents diabetes-mediated GLP-1R nitration and oxidative stress in pericytes
Diabetic conditions significantly increased total nitrotyrosine formation in pericytes (Fig. 2a). HBMPCs grown under normal or diabetic conditions were immunoprecipitated with GLP-1R antibody and then immunoblotted using nitrotyrosine antibody. Our results showed that GLP-1R nitration was significantly increased under diabetic conditions (Fig. 2b) and that treatment with the GLP-1RA exendin-4 reduced the GLP-1R nitration. These results were further confirmed using slot blot analysis, by comparison with epicatechin selective nitration inhibitor (Fig. 2c).
Diabetes mediates GLP-1R nitration and GLP-1RA improves pericyte oxidative defence under diabetic conditions. HBMPCs were grown to confluency in complete media then switched to normal glucose (control) or to high glucose (25 mmol/l) and sodium palmitate (200 μmol/l) to mimic diabetic conditions. (a) Representative western blot and quantification, showing that diabetic conditions significantly increased nitrotyrosine in pericytes (*p<0.05 vs control conditions, n = 3). (b) Representative immunoprecipitation and analysis of GLP-1R nitration. HBMPCs were treated with normal glucose (control) or with high glucose with/without exendin-4 (100 nmol/l). The cellular lysate was immunoprecipitated with GLP-1R antibody and immunoblotted with anti-nitrotyrosine antibody. Diabetic conditions increased GLP-1R nitration (*p<0.05 vs control conditions, n = 3) and treatment with exendin-4 decreased the effect of hyperglycaemia. (c) HBMPCs were treated with normal glucose (control), high glucose with/without epicatechin (10 μmol/l), or high glucose with/without exendin-4 (100 nmol/l). Slot blot analysis of nitrotyrosine formation showed that the nitration inhibitor epicatechin and the GLP-1RA exendin-4 were able to decrease nitrative stress in diabetic conditions (*p<0.05 vs control conditions, n = 5). (d) Diabetes increased production of ROS, as detected using DHE ROS detection kit. Treatment of pericytes with exendin-4 (100 nmol/l) significantly reduced ROS production (*p<0.05 vs control conditions, n = 3). (e) In parallel, the serum from control or diabetic mice was assessed for reduced glutathione levels as a marker for antioxidant defence. Exendin-4 significantly improved antioxidant defence in diabetic mice (*p<0.05 vs control mice, n = 3). (f) HBMPCs were treated with normal glucose (control) or high glucose with/without exendin-4 (100 nmol/l). Nrf-2 gene expression, examined by RT-PCR, was significantly increased by exendin-4 treatment (*p<0.05 vs control conditions, n = 4). (g) MTT cell survival assay showed that diabetic conditions decreased pericyte survival (*p<0.05 vs control conditions, n = 3) and that treatment with exendin-4 increased the pericyte survival under diabetic conditions. Data are presented as mean percentage of control ± SEM. D, diabetes; EPI, epicatechin; Exd-4, exendin-4; GSH, glutathione; HG, high glucose; NG normal glucose; NY, nitrotyrosine; Pal, sodium palmitate
In parallel, diabetic conditions caused a significant increase in intracellular generation of reactive oxygen species (ROS) in the HBMPCs, as detected using dihydroethidium (Fig. 2d); this increase was prevented by exendin-4 treatment. Moreover, levels of glutathione (one of the efficient antioxidant defence systems in the body) were assessed in serum obtained from control mice and diabetic mice treated with/without exendin-4. The diabetic mice showed a decrease in the reduced form of glutathione, when compared with control mice, and treatment with exendin-4 prevented this diabetes-induced oxidative stress (Fig. 2e). We further investigated the effect of exendin-4 on nuclear factor erythroid 2-related factor 2, a transcription factor that regulates the cellular defence. Our results showed that exendin-4 significantly increases Nrf-2 (also known as Nfe2l2) gene expression under diabetic conditions (Fig. 2f).
Increased oxidative stress has been shown to reduce the viability of pericytes [25]. Therefore, we determined pericyte viability using the MTT viability assay kit. Diabetic conditions significantly decreased HBMPC viability; the viability was restored by exendin-4 treatment (Fig. 2g).
GLP-1RA improves pericyte survival signal and decreases inflammation
The pAkt/Akt ratio, a measure of pericyte survival, was reduced in HBMPCs under diabetic conditions (Fig. 3a). Pericyte apoptosis and inflammation was determined by measuring the expression levels of cleaved caspase-3 and inflammatory markers using western blot. Diabetic conditions increased TNF-α, IL-1β and cleaved caspase-3 expression (Fig. 3b–d). Treating the cells with the GLP-1RA exendin-4 significantly reduced the inflammation and apoptosis seen under diabetic conditions.
The GLP-1RA exendin-4 improves pericyte survival signal and decreases inflammation. HBMPCs were grown to confluency in complete media then switched to normal glucose (control), or to high glucose (25 mmol/l) and sodium palmitate (200 μmol/l) with or without exendin-4 (100 nmol/l) for 5 days. Pericyte survival and inflammatory and apoptotic signalling were examined by western blotting. (a) Representative western blot and quantification showing pericyte survival signalling assessed by Akt activation. The pAkt/Akt ratio showed that diabetes decreases pericytes survival. (b, c) Representative western blot and quantification showing pericyte inflammation assessed by TNF-α (b) and IL-1β expression (c). (d) Representative western blot and quantification showing pericyte apoptosis assessed by expression of cleaved caspase-3. Diabetic conditions increased this marker of pericyte apoptosis and this increase was significantly reduced by treating the cells with the GLP-1RA exendin-4. *p<0.05 vs control conditions, n = 4 or 5. Data are presented as mean percentage of control ± SEM. Cl. Casp-3, cleaved caspase-3; Exd-4, exendin-4; HG, high glucose; NG, normal glucose
GLP-1RA improves pericyte function in diabetes
We evaluated the effect of GLP-1RA on pericyte function in diabetes by examining vascular integrity and blood–brain barrier (BBB) function. Diabetes was induced in mice using HFD and low-dose STZ for 8 weeks. The GLP-1RA exendin-4 was administered using an osmotic pump started after 1 month of confirmed diabetes. Diabetic mouse brain sections demonstrated a significant increase in all three vascularisation indices examined (vessel tortuosity, branching density and vascular density) and this increase was prevented by exendin-4 treatment (Fig. 4a).
The GLP-1RA exendin-4 improves pericyte function in diabetes. Diabetes was induced in mice using HFD and low-dose STZ for 8 weeks. The GLP-1RA exendin-4 was administered using an osmotic pump started after 4 weeks of diabetes. Brain sections were stained with Isolectin-B4. Sections were imaged, and 3D reconstructed images were assessed for cerebrovascular neovascularisation indices (vessel tortuosity, branching density and vascular density). (a) Representative images and analysis of neovascularisation indices. Diabetes caused a significant increase in all three vascularisation indices, which were restored by exendin-4. (*p<0.05 vs non-diabetic control, n = 6). Scale bar, 100 μm. (b) Representative immunohistochemistry images and analysis of expression of occludin, a tight junction protein, in mice. Diabetes decreased the expression of occludin compared with control. Treatment with exendin-4 restored occludin expression (*p<0.05, n = 4). Scale bar, 100 μm. (c) Representative western blot and analysis of occludin and VEGF expression in HBMPCs treated with normal glucose (control) or with high glucose with/without exendin-4. Diabetic conditions increased VEGF and decreased occludin expression, and these effects were prevented by exendin-4 (*p<0.05, n = 3 or 4). (d) Representative images for migration assay. A monolayer of HBMPCs was scratched for wound-healing migration assay. Pericytes were treated with either normal glucose or with high glucose with/without exendin-4. Cells exposed to diabetic conditions showed an increase in pericyte migration, when compared with control conditions. Treatment of the pericytes with exendin-4 reduced the diabetes-induced migration (*p<0.05, n = 3). Scale bar, 500 μm. (e) Representative images for migration assay. Cells grown under normal glucose levels were transfected with scrambled siRNA (control) and cells grown under high glucose levels were transfected with scrambled or Glp1r siRNA before being treated with exendin-4. GLP-1R was silenced in pericytes using siRNA. Silencing GLP-1R nullified the protective effect of exendin-4, as evident by the significant increase in migration under diabetic conditions (*p<0.05, n = 3 or 4). Scale bar, 500 μm. Data are presented as mean percentage of control ± SEM. Exd-4, exendin-4; HG, high glucose; NG, normal glucose; Sc, scrambled siRNA
We examined the expression of occludin, an important tight junction protein in the BBB, in control and diabetic mouse brain sections using immunohistochemical studies. Our results showed that diabetic mice displayed significantly lower expression of occludin compared with control mice, and expression levels were restored by treatment with the GLP-1RA exendin-4 (Fig. 4b). Similar findings were made in HBMPCs subjected to diabetic conditions (Fig. 4c). The effect of exendin-4 on the production of vascular endothelial growth factor (VEGF), a potent angiogenic factor, was studied in HBMPCs subjected to diabetic conditions. We found that VEGF production was enhanced under diabetic conditions and was normalised by treating the diabetic cells with exendin-4 (Fig. 4c).
We have previously shown that diabetes increases pericyte migration leading to a decrease in vessel stability and BBB integrity [15, 16]. In the present study, we examined the effect of GLP-1RA on diabetes-induced pericyte migration. HBMPCs were grown under diabetic conditions, and migration was evaluated using a scratch assay. Cells exposed to diabetic conditions showed an increase in pericyte migration compared with control cells, with exendin-4 treatment reducing the diabetes-induced migration (Fig. 4d).
To confirm that decreased migration is due to the activation of GLP-1Rs rather than an intrinsic antioxidant ability of exendin-4, Glp1r was silenced in HBMPCs using siRNA before treatment. Silencing GLP-1R nullified the protective effect of exendin-4, as evident by the significant increase in migration compared with all other treatments (Fig. 4e).
The GLP-1RA exendin-4 improves VCID in diabetic mice
Mouse cognitive function was assessed using Morris water maze and Barnes maze, two well-established models that examine the learning and memory functions in rodents [26, 27]. Our results showed that diabetic mice had significantly impaired learning and memory functions compared with control mice. Treatment with exendin-4 significantly improved the cognitive function of diabetic mice, as assessed by both the water maze (Fig. 5a,b) and Barnes maze (Fig. 5c,d).
The GLP-1RA exendin-4 improves VCID in diabetic mice. Diabetes was induced using HFD and low-dose STZ for 8 weeks. Exendin-4 was administered using an osmotic pump started after 4 weeks of diabetes. Cognitive function was assessed using Morris water maze and Barnes maze. Mice were trained for three consecutive days on each maze then tested on the fourth day. (a, b) Graphical analysis of time spent in (a), and the number of entries into (b), the goal zone in the Water maze. (c, d) Graphical analysis of time spent in (c), and the number of entries into (d), the goal zone in the Barnes maze. Diabetes impaired the learning and memory functions; this impairment was alleviated by exendin-4 treatment. *p<0.05 vs control (non-diabetic mice), n = 5 or 6. Data are presented as mean percentage of control ± SEM. Exd-4, exendin-4
Discussion
The current study provides novel evidence that brain pericytes express the GLP-1R. The main findings of the current study were the following: (1) diabetes increases GLP-1R expression and nitration in pericytes; (2) pericyte dysfunction causes cerebrovascular remodelling and loss of cerebrovascular integrity and BBB function, contributing to VCID in diabetes; (3) GLP-1RA administration helps to restore pericyte function in diabetes by reducing inflammation and oxidative stress, increasing pericyte survival; and (4) restoration of pericyte function contributes to improving cognitive impairment in diabetic mice.
Diabetes is a well-known cause of vascular dementia [28, 29]. Diabetes also increases vascular permeability and immune cell infiltration, damaging the neurons and causing VCID [14, 30, 31]. GLP-1RAs are primarily used in type 2 diabetes treatment [8, 32, 33]. These agents increase insulin secretion in a glucose-dependent manner. Clinical and experimental research shows that GLP-1 RAs have additional beneficial effects in diabetic individuals, such as reducing body weight, decreasing cardiovascular problems and improving cognitive function [6, 8, 32, 34]. Multiple mechanisms have been proposed for the neurovascular protective effects of the GLP-1RAs, including increased insulin secretion and sensitivity, improved hyperglycaemia, enhanced antioxidant defence and decreased neuronal inflammation [9, 17, 28, 33, 34].
In the present study, we propose that the neuroprotective effects of GLP-1RAs arise in part through restoration of lost pericyte function in diabetic brains. Pericytes are heavily involved in maintaining the vasculature and BBB, and regulating angiogenesis [15, 16, 35]. Loss of vascular integrity and BBB breakdown are common events in VCID of different pathogenies such as diabetes, Alzheimer’s disease, post-stroke, ageing and cardiovascular events [36,37,38,39]. GLP-1RAs have been shown to improve the BBB and VCID. Ruze et al showed that GLP-1 contributes to improved cognitive function and brain glucose uptake in diabetic rats through change in GLUT expression in the brain [40]. Xie et al showed that exendin-4 preserves BBB via matrix metallopeptidase 9 (MMP-9) inhibition after subarachnoid haemorrhage [41].
GLP-1Rs are expressed in several cells in the cerebral neurovascular unit, such as endothelial cells, astrocytes and neurons [42,43,44,45,46]. However, the expression and role of GLP-1Rs in brain pericytes has not been previously reported. The current study addressed this gap in knowledge and investigated the contribution made by GLP-1RAs to neurovascular protection in diabetes. First, we used multiple approaches to confirm the expression of GLP-1Rs in pericytes. Moreover, we found that diabetes increased GLP-1R expression in pericytes. Next, we studied the effect of GLP-1R stimulation on pericyte function in diabetes. We and others showed that diabetes causes pericyte dysfunction, resulting in the loss of vascular integrity, increased cerebrovascular pathological neovascularisation and increased pericytes migration [15, 47]. In the current study, we further investigated the role of pericytes in the BBB and vascular integrity. We found that diabetes increases pericyte dysfunction as evidenced by the increase in VEGF expression in pericytes, tight junction protein expression downregulation, and the increase in all indices of pathological neovascularisation. Exendin-4, a GLP-1RA nullified the detrimental impact of diabetes on pericyte function. Our results are in agreement with those of Lin et al who showed that exendin-4 normalised pericyte migration in response to diabetic conditions [47]. These vascular protective effects were greatly reflected by animal cognitive function. Activation of GLP-1Rs in pericytes restored the vascular integrity and significantly improved learning and memory functions of mice as shown by behaviour in the Barnes maze and the Morris water maze.
One of the hallmarks of diabetes is the increase in pericyte death due to increased cellular oxidative stress and inflammation [48]. Our in vitro studies illustrated that exendin-4 significantly decreased ROS production and increased cellular antioxidant defence. Moreover, exendin-4 increased pericyte survival and decreased the expression of inflammatory and apoptotic markers under diabetic conditions. In agreement with our findings, the protective effect of exendin-4 has been demonstrated in various ischaemic disorders. Shan et al showed that exendin-4 could alleviate ischaemia-induced inflammation and BBB breakdown in an astrocyte-dependent manner [17].
Part of the vascular protective effect of GLP-1RAs is attributed to their metabolic effect and improvement of glycaemic control [33]. Although our metabolic findings showed that exendin-4 significantly lowered glucose levels in diabetic mice, our in vitro studies showed that the pericyte protective properties of exendin-4 are independent of glucose, as we showed that treating pericytes with exendin-4 enhanced pericytes survival and reduced inflammation and apoptosis despite the hyperglycaemic conditions.
It is well established in the literature that GLP-1 has neuroprotective effects via reducing neuroinflammation and enhancing signal transduction [49]. The positive impact of GLP-1R stimulation has been demonstrated in various neurological problems, including Alzheimer’s disease and stroke. Interestingly, our results showed an upregulation of GLP-1R expression in vivo and in vitro under diabetic conditions, yet diabetic individuals are at increased risk for neurological problems. Therefore, the next question was to investigate the underlying reasons for the loss of GLP-1R’s protective effect in diabetes. The GLP-1R is a protein that is rich in tyrosine amino acids [50]. Zhang et al showed that the tyrosine moiety in transmembrane domain number one and seven is essential for the receptor function. Any alteration or modification leads to loss of function of the receptor [50]. Therefore, we investigated the post-translational modification of the GLP-1R under diabetic conditions. Our immunoprecipitation results confirmed the nitration of the GLP-1R under hyperglycaemic conditions, possibly explaining the loss of neuroprotective effect of GLP-1Rs in diabetic individuals. In our study, we found that exendin-4 attenuated ROS production, increased oxidative defence and decreased blood sugar, possible mechanisms by which exendin-4 reduces GLP-1R nitration.
Our study has the following limitations: (1) we did not test our hypothesis in GLP-1R knockout mice; (2) we did not address GLP-1R trafficking with treatment; and (3) we did not compare GLP-1RAs against other glucose-lowering agents to isolate the specific effects of central GLP-1R stimulation beyond glycaemic control.
In conclusion, this study provides novel evidence that brain pericytes express the GLP-1R, which is nitrated under diabetic conditions. GLP-1R activation reduces both oxidative and nitrative stress and inhibits the inflammatory and apoptotic pathways in the pericyte under hyperglycaemic conditions. GLP-1R activation improves brain pericyte cell functions that restore vascular integrity and BBB function in diabetes and alleviate diabetes-induced cognitive impairment in mice.
Data availability
Data are available upon request to the corresponding author.
Abbreviations
- BBB:
-
Blood–brain barrier
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1R:
-
GLP-1 receptor
- GLP-1RA:
-
GLP-1R agonist
- HBMPC:
-
Human brain microvascular pericytes cell
- HFD:
-
High-fat diet
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide
- ROS:
-
Reactive oxygen species
- STZ:
-
Streptozotocin
- VCID:
-
Vascular cognitive impairment and dementia
- VEGF:
-
Vascular endothelial growth factor
- 4-VP:
-
4-Vinylpuridine
References
Coucha M, Abdelsaid M, Ward R, Abdul Y, Ergul A (2018) Impact of Metabolic Diseases on Cerebral Circulation: Structural and Functional Consequences. Compr Physiol 8(2):773–799. https://doi.org/10.1002/cphy.c170019
Forbes JM, Fotheringham AK (2017) Vascular complications in diabetes: old messages, new thoughts. Diabetologia 60(11):2129–2138. https://doi.org/10.1007/s00125-017-4360-x
Murphy MP, Corriveau RA, Wilcock DM (2016) Vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta 1862(5):857–859. https://doi.org/10.1016/j.bbadis.2016.02.010
Cukierman-Yaffe T, Gerstein HC, Williamson JD et al (2009) Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 32(2):221–226. https://doi.org/10.2337/dc08-1153
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87(4):1409–1439. https://doi.org/10.1152/physrev.00034.2006
Gonzalez C, Beruto V, Keller G, Santoro S, Di Girolamo G (2006) Investigational treatments for Type 2 diabetes mellitus: exenatide and liraglutide. Expert Opin Investig Drugs 15(8):887–895. https://doi.org/10.1517/13543784.15.8.887
Scheen AJ, Paquot N (2018) Management of hyperglycaemia of type 2 diabetes. Paradigm change according to the ADA-EASD consensus report 2018. Rev Med Liege 73(12):629–633
Palleria C, Leo A, Andreozzi F et al (2017) Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav Brain Res 321:157–169. https://doi.org/10.1016/j.bbr.2017.01.004
Roan JN, Hsu CH, Fang SY et al (2018) Exendin-4 improves cardiovascular function and survival in flow-induced pulmonary hypertension. J Thorac Cardiovasc Surg 155(4):1661–1669 e1664. https://doi.org/10.1016/j.jtcvs.2017.10.085
Pugazhenthi S, Qin L, Reddy PH (2017) Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta 1863(5):1037–1045. https://doi.org/10.1016/j.bbadis.2016.04.017
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7(4):452–464. https://doi.org/10.1215/S1152851705000232
Warmke N, Griffin KJ, Cubbon RM (2016) Pericytes in diabetes-associated vascular disease. J Diabetes Complications 30(8):1643–1650. https://doi.org/10.1016/j.jdiacomp.2016.08.005
Edelman DA, Jiang Y, Tyburski J, Wilson RF, Steffes C (2006) Pericytes and their role in microvasculature homeostasis. J Surg Res 135(2):305–311. https://doi.org/10.1016/j.jss.2006.06.010
Ergul A, Abdelsaid M, Fouda AY, Fagan SC (2014) Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab. https://doi.org/10.1038/jcbfm.2014.18
Coucha M, Barrett AC, Elgebaly M, Ergul A, Abdelsaid M (2019) Inhibition of Ephrin-B2 in brain pericytes decreases cerebral pathological neovascularization in diabetic rats. PLoS One 14(1):e0210523. https://doi.org/10.1371/journal.pone.0210523
Abdelsaid M, Coucha M, Hafez S, Yasir A, Johnson MH, Ergul A (2017) Enhanced VEGF signalling mediates cerebral neovascularisation via downregulation of guidance protein ROBO4 in a rat model of diabetes. Diabetologia 60(4):740–750. https://doi.org/10.1007/s00125-017-4214-6
Shan Y, Tan S, Lin Y et al (2019) The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J Neuroinflammation 16(1):242. https://doi.org/10.1186/s12974-019-1638-6
Abdelwahed OM, Tork OM, Gamal El Din MM, Rashed L, Zickri M (2018) Effect of glucagon-like peptide-1 analogue; Exendin-4, on cognitive functions in type 2 diabetes mellitus; possible modulation of brain derived neurotrophic factor and brain Visfatin. Brain Res Bull 139:67–80. https://doi.org/10.1016/j.brainresbull.2018.02.002
Abdul Y, Abdelsaid M, Li W et al (2019) Inhibition of Toll-Like Receptor-4 (TLR-4) Improves Neurobehavioral Outcomes After Acute Ischemic Stroke in Diabetic Rats: Possible Role of Vascular Endothelial TLR-4. Mol Neurobiol 56(3):1607–1617. https://doi.org/10.1007/s12035-018-1184-8
Abdelsaid M, Prakash R, Li W et al (2015) Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes 64(5):1804–1817. https://doi.org/10.2337/db14-1423
Egholm C, Khammy MM, Dalsgaard T et al (2016) GLP-1 inhibits VEGFA-mediated signaling in isolated human endothelial cells and VEGFA-induced dilation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol 311(5):H1214–H1224. https://doi.org/10.1152/ajpheart.00316.2016
Pang B, Zhou H, Kuang H (2018) The potential benefits of glucagon-like peptide-1 receptor agonists for diabetic retinopathy. Peptides 100:123–126. https://doi.org/10.1016/j.peptides.2017.08.003
Jojima T, Uchida K, Akimoto K et al (2017) Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis 261:44–51. https://doi.org/10.1016/j.atherosclerosis.2017.04.001
Underly RG, Levy M, Hartmann DA, Grant RI, Watson AN, Shih AY (2017) Pericytes as Inducers of Rapid, Matrix Metalloproteinase-9-Dependent Capillary Damage during Ischemia. J Neurosci 37(1):129–140. https://doi.org/10.1523/JNEUROSCI.2891-16.2016
Beltramo E, Porta M (2013) Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem 20(26):3218–3225. https://doi.org/10.2174/09298673113209990022
Barnhart CD, Yang D, Lein PJ (2015) Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One 10(4):e0124521. https://doi.org/10.1371/journal.pone.0124521
Rosenfeld CS, Ferguson SA (2014) Barnes maze testing strategies with small and large rodent models. J Vis Exp 84:e51194. https://doi.org/10.3791/51194
Peng X, Shi X, Huang J et al (2021) Exendin-4 Improves Cognitive Function of Diabetic Mice via Increasing Brain Insulin Synthesis. Curr Alzheimer Res. https://doi.org/10.2174/1567205018666210929150004
Lyu F, Wu D, Wei C, Wu A (2020) Vascular cognitive impairment and dementia in type 2 diabetes mellitus: An overview. Life Sci 254:117771. https://doi.org/10.1016/j.lfs.2020.117771
Mracsko E, Veltkamp R (2014) Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci 8:388. https://doi.org/10.3389/fncel.2014.00388
Fouda AY, Fagan SC, Ergul A (2019) Brain Vasculature and Cognition. Arterioscler Thromb Vasc Biol 39(4):593–602. https://doi.org/10.1161/ATVBAHA.118.311906
Inoue T, Inoguchi T, Sonoda N et al (2015) GLP-1 analog liraglutide protects against cardiac steatosis, oxidative stress and apoptosis in streptozotocin-induced diabetic rats. Atherosclerosis 240(1):250–259. https://doi.org/10.1016/j.atherosclerosis.2015.03.026
Buse JB, Henry RR, Han J et al (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27(11):2628–2635. https://doi.org/10.2337/diacare.27.11.2628
Tai J, Liu W, Li Y, Li L, Holscher C (2018) Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer's disease. Brain Res 1678:64–74. https://doi.org/10.1016/j.brainres.2017.10.012
Harrell CS, Zainaldin C, McFarlane D et al (2018) High-fructose diet during adolescent development increases neuroinflammation and depressive-like behavior without exacerbating outcomes after stroke. Brain Behav Immun 73:340–351. https://doi.org/10.1016/j.bbi.2018.05.018
Cipollini V, Troili F, Giubilei F (2019) Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application. Int J Mol Sci 20(11):2812. https://doi.org/10.3390/ijms20112812
Rosenberg GA (2017) Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond) 131(6):425–437. https://doi.org/10.1042/CS20160604
Geranmayeh MH, Rahbarghazi R, Farhoudi M (2019) Targeting pericytes for neurovascular regeneration. Cell Commun Signal 17(1):26. https://doi.org/10.1186/s12964-019-0340-8
Montagne A, Zhao Z, Zlokovic BV (2017) Alzheimer's disease: A matter of blood-brain barrier dysfunction? J Exp Med 214(11):3151–3169. https://doi.org/10.1084/jem.20171406
Ruze R, Xu Q, Liu G et al (2021) Central GLP-1 contributes to improved cognitive function and brain glucose uptake after duodenum-jejunum bypass on obese and diabetic rats. Am J Physiol Endocrinol Metab 321(3):E392–E409. https://doi.org/10.1152/ajpendo.00126.2021
Xie Z, Enkhjargal B, Nathanael M et al (2021) Exendin-4 Preserves Blood-Brain Barrier Integrity via Glucagon-Like Peptide 1 Receptor/Activated Protein Kinase-Dependent Nuclear factor-Kappa B/Matrix Metalloproteinase-9 Inhibition After Subarachnoid Hemorrhage in Rat. Front Mol Neurosci 14:750726. https://doi.org/10.3389/fnmol.2021.750726
Zheng J, Xie Y, Ren L et al (2021) GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer's disease. Mol Metab 47:101180. https://doi.org/10.1016/j.molmet.2021.101180
Reiner DJ, Mietlicki-Baase EG, McGrath LE et al (2016) Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. J Neurosci 36(12):3531–3540. https://doi.org/10.1523/JNEUROSCI.3579-15.2016
Kawatani M, Yamada Y, Kawatani M (2018) Glucagon-like peptide-1 (GLP-1) action in the mouse area postrema neurons. Peptides 107:68–74. https://doi.org/10.1016/j.peptides.2018.07.010
Shi X, Chacko S, Li F et al (2017) Acute activation of GLP-1-expressing neurons promotes glucose homeostasis and insulin sensitivity. Mol Metab 6(11):1350–1359. https://doi.org/10.1016/j.molmet.2017.08.009
Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK (2011) Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 121(6):2413–2421. https://doi.org/10.1172/JCI43703
Lin WJ, Ma XF, Hao M et al (2018) Liraglutide attenuates the migration of retinal pericytes induced by advanced glycation end products. Peptides 105:7–13. https://doi.org/10.1016/j.peptides.2018.05.003
Wu D, Cederbaum AI (2003) Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 27(4):277–284
Zhao X, Wang M, Wen Z et al (2021) GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front Endocrinol (Lausanne) 12:721135. https://doi.org/10.3389/fendo.2021.721135
Zhang Y, Sun B, Feng D et al (2017) Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546(7657):248–253. https://doi.org/10.1038/nature22394
Acknowledgements
Some of the data were presented as an abstract at the International Stroke meeting in 2020.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All authors contributed to the conception and design of the study, and to the acquisition, analysis and interpretation of data. All authors contributed to drafting the article and gave final approval of the version to be published. MA is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by American Heart Association 16SDG30270013 to MA and a Mercer University Seed grant to MA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 492 kb)
Rights and permissions
About this article
Cite this article
Bailey, J., Coucha, M., Bolduc, D.R. et al. GLP-1 receptor nitration contributes to loss of brain pericyte function in a mouse model of diabetes. Diabetologia 65, 1541–1554 (2022). https://doi.org/10.1007/s00125-022-05730-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05730-5