Abstract
Aims/hypothesis
This study aimed to compare the increase in plasma glucose after a subcutaneous injection of 200 μg glucagon given after 45 min of cycling with resting (study 1) and to investigate the effects of glucagon when injected before compared with after 45 min of cycling (study 2). We hypothesised that: (1) the glucose response to glucagon would be similar after cycling and resting; and (2) giving glucagon before the activity would prevent the exercise-induced fall in blood glucose during exercise and for 2 h afterwards.
Methods
Fourteen insulin-pump-treated individuals with type 1 diabetes completed three visits in a randomised, placebo-controlled, participant-blinded crossover study. They were allocated by sealed envelopes. Baseline values were (mean and range): HbA1c 54 mmol/mol (43–65 mmol/mol) or 7.1% (6.1–8.1%); age 45 years (23–66 years); BMI 26 kg/m2 (21–30 kg/m2); and diabetes duration 26 years (8–51 years). At each visit, participants consumed a standardised breakfast 2 h prior to 45 min of cycling or resting. A subcutaneous injection of 200 μg glucagon was given before or after cycling or after resting. The glucose response to glucagon was compared after cycling vs resting (study 1) and before vs after cycling (study 2).
Results
The glucose response to glucagon was higher after cycling compared with after resting (mean ± SD incremental peak: 2.6 ± 1.7 vs 1.8 ± 2.0 mmol/l, p = 0.02). As expected, plasma glucose decreased during cycling (−3.1 ± 2.8 mmol/l) but less so when glucagon was given before cycling (−0.9 ± 2.8 mmol/l, p = 0.002). The number of individuals reaching glucose values ≤3.9 mmol/l was the same on the 3 days.
Conclusions/interpretation
Moderate cycling for 45 min did not impair the glucose response to glucagon compared with the glucose response after resting. The glucose fall during cycling was diminished by a pre-exercise injection of 200 μg glucagon; however, no significant difference was seen in the number of events of hypoglycaemia.
Trial registration
Clinicaltrials.gov NCT02882737
Funding
The study was funded by the Danish Diabetes Academy founded by Novo Nordisk foundation and by an unrestricted grant from Zealand Pharma
Similar content being viewed by others

Introduction
Exercise can be challenging for individuals with type 1 diabetes. There is an increased risk of hypoglycaemia during the activity and for several hours afterwards, and the increased risk of nocturnal hypoglycaemia can persist for days [1]. Aerobic exercise also reduces total sleep duration the following night by an average of 70 min, probably because of nocturnal hypoglycaemia [2].
The increased risk of hypoglycaemia during exercise is multifactorial. Exercise increases glucose uptake into muscle cells independently of insulin [3]. In healthy individuals, the subsequent decrease in plasma glucose mediates a reduction in insulin secretion and an increase in glucagon levels [4]. The resulting change in the insulin/glucagon ratio in the portal vein is important for hepatic glucose output, which increases and matches the exercise-induced glucose uptake [5]. In individuals with type 1 diabetes, insulin is provided exogenously, and physiological downregulation of insulin secretion cannot occur. This leaves individuals in an unwanted hyperinsulinaemic state. Furthermore, their glucagon response to exercise and hypoglycaemia is attenuated [6, 7]. Moreover, in individuals with type 1 diabetes, the sympathoadrenal response is also attenuated and is further reduced after exercise [7].
Guidelines for avoiding hypoglycaemia during exercise include carbohydrate intake and reduction of bolus and/or basal insulin before, during and after exercise [8, 9]. The exact adjustments needed in relation to exercise are complex, individual and need to be implemented well in advance to allow for circulating insulin levels to drop by the start of exercise [10].
While mild hypoglycaemia may be prevented and treated by intake of carbohydrates, an injection of 1 mg glucagon treats severe hypoglycaemia effectively [11]. Lower doses of subcutaneous glucagon have also been shown to treat mild hypoglycaemia effectively [12, 13] and also to manage children with type 1 diabetes during episodes of impending hypoglycaemia due to gastroenteritis or poor oral intake of carbohydrates [14]. A recently published study found that an injection of 150 μg glucagon may be more effective than insulin reduction for preventing exercise-induced hypoglycaemia and may also result in less post-intervention hyperglycaemia (i.e. less time spent in hyperglycaemia and fewer hyperglycaemic episodes) than ingestion of carbohydrates [15]. Currently, the use of low doses of glucagon to manage mild hypoglycaemia is not recommended by official guidelines for type 1 diabetes. The main obstacle is that glucagon for injection is currently available in a powder form that needs to be reconstituted immediately before use. However, pharmaceutical companies are now developing more convenient glucagon formulations, including powder for nasal administration and soluble formulations for injecting [16, 17]. Another obstacle is that lower doses of glucagon have shown to be less effective for treating hypoglycaemia in adults eating a low-carbohydrate diet compared with those eating a high-carbohydrate diet [18]. One study concluded that the ability of lower doses of glucagon to treat mild hypoglycaemia persisted with preceding ethanol intake, although it tended to be attenuated [19]. Other factors that may affect the efficacy of low doses of glucagon need further exploration.
In this in-clinic study, we compared the glucose-elevating effect of low-dose subcutaneous glucagon given before and after moderate aerobic exercise (cycling) and after resting in individuals with type 1 diabetes. We hypothesised that: (1) the glucose response to glucagon would be similar after cycling and resting; and (2) the exercise-induced fall in blood glucose would be prevented during and for 2 h after exercise with glucagon given before the activity.
Methods
A randomised, placebo-controlled, single-blinded, crossover study was performed. Study participants gave informed consent before inclusion. The study involved two substudies; one comparing the glucose response to glucagon after cycling vs after resting (primary study; study 1); and another comparing the glucose response to glucagon before or after cycling (study 2). The study was monitored by the Good Clinical Practice Unit at Copenhagen University Hospital Bispebjerg, Denmark and approved by the Danish Health and Medicines Authority (EudraCT 2016-002127-28), the Regional Committee on Health Research Ethics (H-16016762) and the Danish Data Protection Agency (2012-58-0004). It was conducted in accordance with the Helsinki Declaration and was registered (Clinicaltrials.gov registration no. NCT02882737).
Recruitment
Participants were recruited from the outpatient diabetes clinic at Copenhagen University Hospital Hvidovre in the period September 2016 to May 2017. The inclusion criteria were: age 18–70 years; BMI 20–30 kg/m2; HbA1c ≤69 mmol/mol (≤8.5%); duration of type 1 diabetes ≥2 years; insulin pump treatment ≥1 year; self-reported hypoglycaemia awareness; use of carbohydrate counting for all meals; and use of the insulin pump bolus calculator for all meals. Participants were excluded if they were known to be intolerant to glucagon or lactose, were pregnant or breastfeeding, performed vigorous intense aerobic exercise for more than 3 h per week or used medication influencing glucose metabolism (in addition to insulin).
The study was conducted at the research unit at the Department of Endocrinology at Copenhagen University Hospital Hvidovre. The participants went through a screening day, a run-in period and then, in random order, three different visits.
Screening
The screening was performed after an overnight fast. Information was collected on sex, age, race, diabetes duration, allergies, medical history and medications. Height, weight, blood pressure and heart rate were measured; questionnaires to identify hypoglycaemia unawareness [20, 21] were filled out and a 12-lead electrocardiogram was performed. The duration of insulin pump use, insulin pump settings and total daily insulin dose were recorded. Orthostatic hypotension and beat-to-beat variations were determined [22]. Residual beta cell function was assessed with an intravenous glucose glucagon test [23]. Information about the participants’ current exercise habits was obtained using the General Practice Physical Activity Questionnaire (GPPAQ) [24]. Prior to visits, participants’ insulin pump settings (basal settings, carbohydrate-to-insulin ratios, insulin sensitivities and insulin action time) were adjusted systematically over a 2–3 week period [25]. Around 48 h before the visits, a continuous glucose monitor (CGM) (Dexcom G4, Platinum, San Diego, CA, USA) was placed on the abdominal wall at least 7 cm from the site of the insulin pump infusion set. The CGM results were used to exclude any episode of hypoglycaemia (≤3.9 mmol/l) for 12 h before the visit. Avoidance of hypoglycaemia during the night preceding a visit was secured by CGM and self-monitoring of blood glucose. If a sensor value fell below 3.9 mmol/l an alarm would be activated, but, in all cases, self-monitoring of blood glucose confirmed values higher than 3.9 mmol/l. Further, pedometers were provided (Omron Walking Style Pro 2.0 HJ-322 U-E, Omron Dalian, Dalian, China) to estimate participants’ activity level 2 days prior to each visit. All participants used their own insulin pump and did not change their pump settings between visits. The abdominal wall was chosen for the insulin pump infusion sites. Glucagon was injected subcutaneously in the abdominal wall at least 6 cm from the CGM and insulin infusion site.
Visits
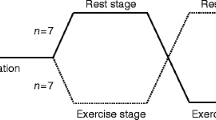
The study design is summarised in Fig. 1. At all visits, participants arrived at 08:00 hours after an overnight fast. They were instructed to aim for plasma glucose close to 6.0 mmol/l on arrival. They measured capillary blood glucose at 03:00 hours to decide on a last bolus/carbohydrate intake at that time. An antecubital vein catheter was positioned and covered with a heating pad. Before the study started, initial blood and urine samples were taken to measure plasma glucose, plasma glucagon, plasma ketones, serum NEFA, serum triacylglycerols and, for fertile women, urine human chorionic gonadotropin. Participants were served a meal counting 40 g carbohydrates (30 g carbohydrates from rye bread with butter and cheese and 10 g carbohydrates from milk or juice). Before starting the meal, participants used their insulin pump to calculate and administer a meal bolus (correction insulin included, blood glucose target 5.0 mmol/l) (t = 0). After 130 min, participants were instructed to either cycle (two visits) or rest (one visit) for 45 min or until a plasma glucose ≤3.9 mmol/l was reached. There were at least 6 days between visits.
The experimental design. A randomised, placebo-controlled, participant-blinded crossover study. Participants went through three visits at which they consumed a standardised breakfast and were observed for 130 min. The first bolus of either saline (placebo) or glucagon (200 μg) was injected subcutaneously before 45 min of cycling or resting. Thereafter, the second bolus of either glucagon or placebo was given and participants then rested for 120 min. Study 1: glucagon after cycling (b) vs glucagon after resting (a). Study 2: glucagon after cycling (b) vs glucagon before cycling (c)
Visits with exercise
Participants cycled with a heart rate equal to 50% of their heart rate reserve (HRmax). HRmax was calculated as 220 − age. We chose 50% HRmax to standardise exercise between the visits and to ensure that the individuals performed aerobic exercise. The heart rate levels were calculated using the formula of Karvonen et al [26]: HRstudy = 50% × (HRmax − HRrest) + HRrest. The heart rate was measured continuously with a heart rate monitor (Polar Wearlink RS300X, Polar Electro, Kempele, Finland). The resistance of the ergometer was adjusted to reach HRstudy. Subcutaneous injections of either 0.2 ml isotonic saline (154 mmol/l NaCl) or 200 μg glucagon were given in random order before or after cycling. The choice of a fixed dose of 200 μg was made because of the desire to increase participants’ blood glucose levels by 3–5 mmol/l and, further, there would be no effect of body weight on the glucose-increasing effect of glucagon [13]. If the individual reached plasma glucose ≤3.9 mmol/l during cycling then the cycling was stopped and the individual was given either a glucagon or saline injection.
Visits with resting
Participants rested on a hospital bed. A subcutaneous injection of 0.2 ml isotonic saline was given before resting for 45 min and a 200 μg glucagon injection was given afterwards. If an individual’s plasma glucose fell to ≤2.5 mmol/l then the study day was stopped and carbohydrates given.
Measurements
Blood samples were analysed for plasma glucose, plasma lactate, plasma glucagon, plasma ketones, serum insulin aspart, serum NEFA and serum triacylglycerol, as described previously [13, 18]. We measured the total insulin concentration without antibody precipitation. We monitored the blood pressure and heart rate, made clinical assessments and further assessed adverse events (nausea, stomach pain, headache, dizziness and hunger) using a 100 mm visual analogue scale (VAS) with extremes at each end. Vomiting, palpitation or sweating events were also recorded.
Randomisation and blinding
Participants were randomly assigned to one of six different study sequences (a = glucagon after resting, b = glucagon after cycling, c = glucagon before cycling: abc, acb, bca, etc.) by opening a sealed envelope containing a randomisation code. The sealed envelopes had been prepared by a person not otherwise involved in the study. On each visit, 5–15 min before the cycling and resting periods, the primary investigator prepared a syringe containing either 0.2 ml (200 μg) glucagon or 0.2 ml saline. The participants were blinded to the injections on cycling visits but not on resting visits.
Study outcomes
The primary endpoint was the difference in incremental plasma glucose peak after 200 μg subcutaneous glucagon given after either cycling or resting. Secondary endpoints were difference in plasma glucose change during cycling with or without pre-cycling glucagon, difference in time-to-plasma glucose peak after glucagon injection with or without cycling, the total (t)AUC after each glucagon injection, biochemical changes after each glucagon injection (ketones, lactate, glucagon, NEFA and triacylglycerols) and number of events of hypoglycaemia (plasma glucose ≤3.9 mmol/l during and 2 h after exercise and resting) in the three study groups.
Statistical analysis
The primary aim was to compare the incremental peak plasma glucose produced by glucagon after exercise vs after resting. In the third study arm, glucagon was given before these activities, which we consider a separate experiment. Therefore, we chose to perform a power calculation based on the post-exercise/resting arms. According to our calculations, fourteen participants should be included (Student’s t test) to be able to detect a difference in incremental peak plasma glucose (primary outcome) between the two study arms of 1.0 mmol/l with 80% power, a 5% significance level and a 0.8 mmol/l (within and between variation) standard deviation. The sample size calculation was based on data obtained from a previous study of low-dose glucagon administration in a similar population [18]. The data on incremental peak plasma glucose after glucagon injection were logarithmically transformed to obtain normal distribution. A paired Student’s t test was used to compare the logarithmic incremental peak plasma glucose levels after cycling and after resting. For comparisons of secondary outcomes between the three study arms, a repeated-measurement ANOVA (RM-ANOVA) was used and multiple comparisons were Bonferroni adjusted. We used SAS 9.4 (SAS Institute, NC, USA) and GraphPad Prism 7.0 (GraphPad Software, CA, USA) for the statistical analyses and considered p < 0.05 as statistically significant. If not otherwise stated, calculated data are presented as mean (SEM) and baseline characteristics as median (range).
Results
Participants
Eighteen individuals participated in a screening visit, but four withdrew from the study because of a lack of time to complete the subsequent visits. Fourteen individuals performed the two visits needed for the primary data analysis. One participant became pregnant before the visit at which glucagon was to be given before cycling and was excluded from the secondary data analysis.
Baseline data
The 14 participants (six women) were 47 years of age (23–66 years), had had type 1 diabetes for 26 years (8–51 years), had an HbA1c of 54 mmol/mol (43–65 mmol/mol) or 7.1% (6.1–8.1%), BMI 26 kg/m2 (21–30 kg/m2), used insulin pumps for 7 years (1–35 years) and had a total daily insulin dose of 34 U (16–57 U). The basal insulin dose was 55% of the total daily dose and the bolus insulin dose was 45% of the total daily dose. The participants used their own insulin pumps throughout the study and did not change insulin pump settings between visits. One participant was Asian and the others were white. Partial hypoglycaemia unawareness was identified for three participants by Gold’s methods [20] and six participants by Clarke’s method [22]. One participant was identified as physically inactive, four were moderately inactive, six moderately active and three were active by GPPAQ [24]. No participants had orthostatic hypotension, but nine participants had impaired beat-to-beat variation. Residual beta cell function was negligible in 13 participants (stimulated C-peptide level <60 pmol/l) but one participant had a stimulated C-peptide level of 74 pmol/l. Biochemical and haematological markers were within normal range at screening (Table 1). The number of steps performed 24 h before the visits did not differ between the three visits (glucagon after resting 6454 steps, glucagon after cycling 7071 steps and glucagon before cycling 7034 steps; p = 0.44).
Plasma glucose
The fasting mean plasma glucose did not differ between the visits with glucagon before cycling, glucagon after cycling and glucagon after resting (8.1 ± 2.2 vs 8.8 ± 3.6 vs 9.3 ± 2.6 mmol/l, respectively; p = 0.57). There was a higher incremental plasma glucose peak when glucagon was given after 45 min of cycling compared with after resting (2.6 ± 1.7 vs 1.8 ± 2.0 mmol/l, respectively; p = 0.02) (Fig. 2a, Table 2). The time to peak (36.4 vs 42.5 min) and the tAUC (1465.2 vs 1786.1 mmol/l × min) were similar from 0 to 120 min after the glucagon injections on both visits (all p > 0.05). Plasma glucose levels decreased less during cycling when glucagon was given before cycling compared with saline (visit with glucagon after cycling) (−0.9 ± 2.8 vs −3.1 ± 2.8 mmol/l; p = 0.002).
Concentrations of plasma glucose (a), plasma glucagon (b) and serum insulin aspart (c). Data are presented as mean ± SEM for three visits starting with a postprandial period of 130 min, followed by glucagon before cycling, glucagon after cycling or glucagon after resting. The area between the vertical dotted lines represents the cycling/resting period. Glucagon resulted in a higher incremental plasma glucose peak when given after 45 min of cycling compared with after resting (p = 0.02). There were no differences between visits in peak glucagon levels (p = 0.29). There were no differences in peak serum insulin aspart levels (p = 0.43)
There was no significant difference in the number of events of plasma glucose ≤3.9 mmol/l between the two cycling visits during and in the observation period after cycling, whether glucagon was given before or after cycling (in total 6 vs 4, p = 0.41). On the resting visit, two participants experienced plasma glucose ≤3.9 mmol/l (p = 0.14 compared with the cycling visits). The duration of cycling before plasma glucose ≤3.9 mmol/l was longer when glucagon was given before compared with after cycling (p = 0.0065).
Plasma glucagon
Fasting glucagon levels did not differ between visits (p = 0.29). There were no differences between visits regarding peak glucagon levels (p = 0.29) or time to peak after the glucagon injection (p = 0.21) (Fig. 2b, Table 2). The change in endogenous glucagon levels did not differ during cycling compared with resting (p = 0.5) (electronic supplementary material [ESM] Fig. 1).
Serum insulin aspart
Fasting serum insulin levels were higher on the day glucagon was given before compared with after cycling and after resting (89.6 ± 11.1 vs 63.2 ± 10.4 pmol/l, p = 0.01, and 74.3 ± 13.2 pmol/l, p = 0.04; overall p = 0.04). Insulin doses given at breakfast did not differ between visits (p = 0.93). There were no differences in peak insulin levels (p = 0.43) or time to peak after insulin injections in all three visits (p = 0.24) (Fig. 2c, Table 2). Serum insulin aspart levels tended to decrease less during cycling than during resting (p = 0.07) (ESM Fig. 1).
Plasma lactate
Fasting plasma lactate levels did not differ between visits (p = 0.94). Peak plasma lactate levels were significantly higher on both cycling visits compared with the resting visit (p < 0.0001) (Fig. 3a, Table 2). However, no differences were seen between the cycling visits (p = 0.99).
Concentrations of plasma lactate (a), plasma ketones (b), serum triacylglycerols (c) and serum NEFA (d). Data are presented as mean ± SEM for the three visits, starting with a postprandial period of 130 min, followed by glucagon before cycling, glucagon after cycling or glucagon after resting. The area between the vertical dotted lines represents the cycling/resting period
Plasma ketones
Fasting plasma ketone levels were similar between visits (p = 0.26) (Fig. 3b). Plasma ketones were similar during cycling compared with resting (p = 0.18) but became significantly higher 2 h after cycling compared with resting (p < 0.001).
Serum triacylglycerols
Serum triacylglycerol levels over time did not differ between visits (p = 0.28; Fig. 3c).
Serum NEFA
Fasting serum NEFA levels were similar between visits (p = 0.07; Fig. 3d). Serum NEFA level after the resting session was lower than after the two cycling sessions (p = 0.02) and remained lower 2 h after resting compared with cycling (p < 0.0001).
Adverse events
There was no difference in VAS score for nausea over time between visits (p = 0.6). However, one participant consistently reported nausea (mean VAS increase 2.83 cm) after glucagon injection in all three visits. No participants reported vomiting, palpitation or excessive sweating.
Discussion
To our knowledge, this is the first study comparing the glucose responses to glucagon given after cycling and after resting. We found that the maximal glucose response after 200 μg glucagon was highest when given after 45 min of cycling compared with after 45 min of resting.
The difference might be related to an exercise-induced increase in gluconeogenic precursors. Central in gluconeogenesis is lactate, the levels of which were elevated equally and by a factor of three after the two exercise sessions. Other possible gluconeogenic precursors are glycerol, alanine and glutamine [27], which are most likely increased by exercise but were not measured in the present study. However, the increase in ketones and NEFA levels documented during both exercise days are likely to parallel glycerol levels. Another explanation for the increased response to glucagon after exercise may be an increased absorption of glucagon due to increased subcutaneous blood flow during exercise compared with during resting. The overall glucose response to glucagon, measured as tAUC, did not differ between the visits, leading to the conclusion that glucose response to low-dose glucagon is preserved after moderate exercise compared with resting conditions. Exercise diminishes liver glycogen stores by similar rates in healthy individuals and those with type 1 diabetes [28]. Because the duration of cycling was relatively small and considering the intake of breakfast 2 h prior to cycling, the risk of depletion of liver glycogen is likely to have been small [29, 30]. Measurements of hepatic glycogen stores would have been valuable for a full explanation of our results. Among other explanations for this finding could be an exercise-induced increase in glucagon sensitivity by upregulation of the liver glucagon receptors as described in animal studies [31, 32].
We found no increase in endogenous glucagon levels during cycling compared with resting, which may partly explain the increased risk of hypoglycaemia during exercise for individuals with type 1 diabetes. Furthermore, a brief initial increase in insulin aspart levels was observed during the cycling period during both visits with cycling and this could also explain the increased risk of hypoglycaemia [33].
We did not see a significant difference in the serum concentrations of insulin aspart between resting and cycling visits that could have been responsible for a different glucose response to glucagon. Presumably, exercise increases the absorption of subcutaneous insulin delivery through minor mechanical displacements or increased blood flow in the subcutaneous tissue during exercise [8]. Inappropriate glucagon and insulin excursions during exercise have previously been demonstrated in individuals with type 1 diabetes [6]. Because of this, administration of glucagon before exercise might be expected to prevent or reduce hypoglycaemia. Indeed, the glucagon injection markedly reduced the exercise-induced decrease in plasma glucose. There was no statistical difference in the rates of hypoglycaemia between the three visits, regardless of giving glucagon before or after the cycling. The number of hypoglycaemic events was, however, small, which is why a larger study with enough power to compare the hypoglycaemia rates between the visits is needed. Moreover, the pre-exercise plasma glucose values were, for unknown reasons, significantly lower on the day with pre-exercise glucagon, which probably affected the number of readings at 3.9 mmol/l.
The pre-exercise prandial insulin bolus dose was not reduced as stated in international guidelines [8]. This approach was chosen to study the effects of glucagon in case of unplanned exercise. Therefore, the active musculature was exposed to relative hyperinsulinaemia. Even though glucose disposal during exercise is higher than during resting, glucagon still managed to produce similar incremental plasma glucose after exercise and resting. We chose the subcutaneous insulin method to better simulate real-life events, but using an i.v. clamp method could have avoided this bias [34]. Recently, results were published from a study in a similar group of individuals performing 45 min of exercise at four different sessions under conditions of: (1) no intervention; (2) 50% basal insulin reduction; (3) 40 g oral glucose tablets; or (4) after injections of 150 μg glucagon [15]. In contrast to our study, the investigators found that none of the participants receiving glucagon before exercise experienced hypoglycaemia. In that study, several conditions differed from ours: the participants had higher plasma glucose before the exercise session; they did not eat breakfast 120 min before exercise; another type and dose of glucagon was used; and the glucagon was given 5 min before the exercise started, while we gave glucagon immediately before cycling. Based on their result, Rickels and co-workers concluded that glucagon injections may be more effective than insulin reduction for preventing exercise-induced hypoglycaemia. Our data support the possibility of using glucagon to prevent the decrease in plasma glucose during exercise.
Our study had limitations. It was conducted in a controlled inpatient environment; therefore, future trials in real-life settings will be needed to confirm our results. Further, our data are based on individuals with longstanding type 1 diabetes and near-normal glucose control. The glucagon effect on plasma glucose might have been different in individuals performing anaerobic exercise or aerobic exercise for different durations or intensities. Furthermore, individuals with other exercise habits—athletes or wholly sedentary individuals—may respond differently to glucagon after exercise. We used a rather crude estimate of Vmax (heart rate) but found no difference in peak lactate between the two exercise visits, indicating that the exercise intensities were similar. A single dose of 200 μg glucagon was used in this study. One could argue as well for other doses of glucagon before exercise to find the best dose to prevent hypoglycaemia during cycling [13]. Another limitation is that insulin resistance mediated by catecholamines and cortisol release in response to exercise may have enhanced the glucose response to glucagon administration as well as directly increasing the release of gluconeogenic precursors. However, these counter-regulatory hormones were not measured.
The strongest barrier to regular exercise in adults with type 1 diabetes is the fear of hypoglycaemia [35]. Several strategies are available to prevent exercise-induced hypoglycaemia: carbohydrate intake; reduction in meal insulin bolus before exercise [8]; manual reduction of basal insulin infusion before exercise [36]; and using a sensor-augmented insulin pump with predictive low glucose management (PLGM) that allows for automated suspension of basal insulin delivery in response to a CGM-predicted low glucose level [37]. Our study points to other possibilities for hypoglycaemia prevention and treatment during exercise. A possible strategy for avoiding exercise-induced hypoglycaemia could be the combined use of mini-dose glucagon together with insulin pumps with PLGM or insulin pens with CGMs. Earlier studies have shown that low-dose glucagon is effective in treating hypoglycaemia as an add-on to insulin in both open-loop [13] and closed-loop settings [38]. Our data further indicate that moderate exercise of 45 min does not impair the glycaemic response of low-dose glucagon. We could not, however, demonstrate a significant reduction in hypoglycaemic events by using pre-exercise glucagon injections compared with post-exercise glucagon injections.
In conclusion, the glucose response to glucagon after 45 min cycling was preserved and, surprisingly, even greater compared with the glucose response after resting. The decrease in blood glucose during cycling was slightly diminished by adding a glucagon injection before 45 min cycling, but the occurrence of hypoglycaemic events during and after cycling was not prevented in the current study. The preserved effect on glucose suggests that low-dose glucagon should be studied in real-life setting for the effectiveness in treating mild hypoglycaemia in the hours following moderate exercise.
Data availability
Datasets may be requested from the corresponding author. For the full trial protocol, email the corresponding author.
Abbreviations
- ALAT:
-
Alanine aminotransferase
- CGM:
-
Continuous glucose monitor
- GPPAQ:
-
General Practice Physical Activity Questionnaire
- HR:
-
Heart rate
- HRmax :
-
HR reserve calculated as 220 – age
- HRrest :
-
HR at resting
- HRstudy :
-
HR during cycling calculated as 50% × (HRmax − HRrest) + HRrest
- PLGM:
-
Predictive low glucose management
- tAUC:
-
Total AUC
- VAS:
-
Visual analogue scale
References
Bolli GB, Dimitriadis GD, Pehling GB et al (1984) Abnormal glucose counterregulation after subcutaneous insulin in insulin-dependent diabetes mellitus. N Engl J Med 310(26):1706–1711. https://doi.org/10.1056/NEJM198406283102605
Reddy R, El Youssef J, Winters-Stone K et al (2018) The impact of exercise on sleep in adults with type 1 diabetes. Diabetes Obes Metab 20(2):443–447. https://doi.org/10.1111/dom.13065
Ploug T, Galbo H, Richter E (1984) Increased muscle glucose uptake during contractions: no need for insulin. Am J Phys 247(October):E726–E731
Tansey MJ, Tsalikian E, Beck RW et al (2006) The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes. Diabetes Care 29:20–25
Wasserman DH, Williams PE, Lacy DB, Goldstein RE, Cherrington AD (1989) Exercise-induced fall in insulin and hepatic carbohydrate metabolism during muscular work. Am J Phys 256:E500–E509
Mallad A, Hinshaw L, Schiavon M et al (2015) Exercise effects on postprandial glucose metabolism in type 1 diabetes: a triple-tracer approach. Am J Physiol Endocrinol Metab 308(12):E1106–E1115. https://doi.org/10.1152/ajpendo.00014.2015
Cryer PE (2009) Exercise-related hypoglycemia-associated autonomic failure in diabetes. Diabetes 58(9):1951–1952. https://doi.org/10.2337/db09-0834
Riddell MC, Gallen IW, Smart CE et al (2017) Review exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 8587:1–14
Pinsker JE, Kraus A, Gianferante D et al (2016) Techniques for exercise preparation and management in adults with type 1 diabetes. Can J Diabetes 40(6):503–508. https://doi.org/10.1016/j.jcjd.2016.04.010
Zaharieva DP, Riddell MC (2017) Insulin management strategies for exercise in diabetes. Can J Diabetes 41(5):507–516. https://doi.org/10.1016/j.jcjd.2017.07.004
Collier A, Steedman DJ, Patrick AW et al (1987) Comparison of intravenous glucagon and dextrose in treatment of severe hypoglycemia in an accident and emergency department. Diabetes Care 10(6):712–715. https://doi.org/10.2337/diacare.10.6.712
Haymond MW, Redondo MJ, McKay S et al (2016) Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults with type 1 diabetes: a dose-seeking study. Diabetes Care 39(3):465–468. https://doi.org/10.2337/dc15-2124
Ranjan A, Schmidt S, Madsbad S, Holst JJ, Nørgaard K (2016) Effects of subcutaneous, low-dose glucagon on insulin-induced mild hypoglycaemia in patients with insulin pump treated type 1 diabetes. Diabetes Obes Metab 18(4):410–418. https://doi.org/10.1111/dom.12627
Haymond MW, Schreiner B (2001) Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care 24(4):643–645. https://doi.org/10.2337/diacare.24.4.643
Rickels M, Dubose S, Wolpert H et al (2018) Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes. Diabetes Care 41(9):1909–1916. https://doi.org/10.2337/dc18-0051
Hövelmann U, Bysted BV, Mouritzen U et al. (2017) Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care: 41:531–537. https://doi.org/10.2337/dc17-1402
Sherr JL, Ruedy KJ, Foster NC et al (2016) Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care 39(4):555–562. https://doi.org/10.2337/dc15-1606
Ranjan A, Schmidt S, Damm-Frydenberg C et al (2017) Low-carbohydrate diet impairs the effect of glucagon in the treatment of insulin-induced mild hypoglycemia: a randomized crossover study. Diabetes Care 40(1):132–135. https://doi.org/10.2337/dc16-1472
Ranjan A, Nørgaard K, Tetzschner R et al (2018) Effects of preceding ethanol intake on glucose response to low-dose glucagon in individuals with type 1 diabetes: a randomized, placebo-controlled, crossover study. Diabetes Care 41(4):797–806. https://doi.org/10.2337/dc17-1458
Gold AE, MacLeod KM, Frier BM (1994) Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 17(7):697–703. https://doi.org/10.2337/diacare.17.7.697
Pedersen-Bjergaard U, Pramming S, Heller SR et al (2004) Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev 20(6):479–486. https://doi.org/10.1002/dmrr.482
Høi-Hansen T, Pedersen-Bjergaard U, Thorsteinsson B (2010) Classification of hypoglycemia awareness in people with type 1 diabetes in clinical practice. J Diabetes Complicat 24(6):392–397. https://doi.org/10.1016/j.jdiacomp.2009.07.006
Scheen AJ, Castillo MJ, Lefèbvre PJ (1996) Assessment of residual insulin secretion in diabetic patients using the intravenous glucagon stimulatory test: methodological aspects and clinical applications. Diabetes Metab 22(6):397–406
UK Department of Health and Social Care (2013) The General Practice Physical Activity Questionnaire (GPPAQ) report. Department of Health, London, p 2006 Available from: www.gov.uk/government/publications/general-practice-physical-activity-questionnaire-gppaq
Walsh J, Roberts R, Bailey T (2011) Guidelines for optimal bolus calculator settings in adults. J Diabetes Sci Technol 5(1):129–135. https://doi.org/10.1177/193229681100500118
Karvonen MJ, Kentala E, Mustala O (1957) The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 35(3):307–315
Gerich JE, Woerle HJ, Meyer C, Stumvoll M (2001) Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24(2):382–391. https://doi.org/10.2337/diacare.24.2.382
Petersen KF, Price TB, Bergeron R (2004) Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. J Clin Endocrinol Metab 89(9):4656–4664. https://doi.org/10.1210/jc.2004-0408
Greenhaff PL, Hultman E, Harris RC (2004) Carbohydrate metabolism. In: Poortmans JR (ed) Principles of exercise biochemistry, 3rd, rev ed. Med Sport Sci. Basel, Karger, vol 46, Brussels, pp 133
Bélanger P, Couturier K, Latour MG, Lavoie JM (2000) Effects of supranormal liver glycogen content on hyperglucagonemia-induced liver glycogen breakdown. Eur J Appl Physiol 83:328–335
Lavoie C (2005) Glucagon receptors: effect of exercise and fasting. Can J Appl Physiol 30(3):313–327. https://doi.org/10.1139/h05-124
Melançon A, Gagnon V, Milot M et al (2013) Liver glucagon receptors (GluR): effect of exercise and fasting on binding characteristics, GluR-mRNA, and GluR protein content in rats. Horm Metab Res 45(10):716–721. https://doi.org/10.1055/s-0033-1349853
Schmidt S, Finan DA, Duun-Henriksen AK et al (2012) Effects of everyday life events on glucose, insulin, and glucagon dynamics in continuous subcutaneous insulin infusion-treated type 1 diabetes: collection of clinical data for glucose modeling. Diabetes Technol Ther 14(3):210–217. https://doi.org/10.1089/dia.2011.0101
Ranjan A (2018) Glucagon treatment in type 1 diabetes -with focus on restoring plasma glucose during mild hypoglycemia. Dan Med J 65:B5449
Brazeau A-S, Rabasa-Lhoret R, Strychar I, Mircescu H (2008) Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 31(11):2108–2109. https://doi.org/10.2337/dc08-0720
Zaharieva D, Yavelberg L, Jamnik V, Cinar A, Turksoy K, Riddell MC (2017) The effects of basal insulin suspension at the start of exercise on blood glucose levels during continuous versus circuit-based exercise in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Technol Ther 19(6):370–378. https://doi.org/10.1089/dia.2017.0010
Steineck I, Ranjan A, Nørgaard K, Schmidt S (2017) Sensor-augmented insulin pumps and hypoglycemia prevention in type 1 diabetes. J Diabetes Sci Technol 11(1):50–58. https://doi.org/10.1177/1932296816672689
Russell SJ, El-Khatib FH, Sinha M et al (2014) Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 371(4):313–325. https://doi.org/10.1056/NEJMoa1314474
Acknowledgements
The authors thank the study participants and acknowledge the laboratory assistance by A. Sloth Andersen (Department of Endocrinology Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark) and J. Bjerager and J. Laigard (medical students, University of Copenhagen). Furthermore, we thank L. Brus Albæk (Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark) for analysing the glucagon, R. Raml (Joanneum Research, Graz, Austria) for the aspart analysis, J. Nymann (Department of Biochemistry, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark) for analysing triacylglycerols and NEFA, and G. Kølander Hansen (Obesity Research, Novo Nordisk, Måløv, Denmark) for measuring plasma total ketones.
Some of the data were presented at the Advanced Technologies and Treatments for Diabetes (ATTD) meeting, Vienna Austria and the EASD meeting, Berlin, Germany, in 2018.
Funding
This study was funded by the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation, and by an unrestricted grant from Zealand Pharma. Dexcom provided transmitters, receivers and sensors for the study. The Danish Diabetes Academy, Zealand Pharma and Dexcom hold no rights to the study results. The study sponsors were not involved in the design of the study, the collection, analysis, or interpretation of data, writing the report or the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
IIKS, AR, SS and KN conceived the idea and designed the study. IIKS performed the studies, analysed the data and wrote and edited the manuscript. AR performed some of the studies, analysed the data and reviewed and edited the manuscript. JJH and TRC provided data and reviewed, edited and approved the final manuscript. SS and KN reviewed, edited and approved the final manuscript. IIKS, AR and KN take full responsibility for the content of this article and are responsible for the integrity of the work as a whole. All authors made substantial contributions to conception and design of the study, acquisition of data or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content and all gave final approval of the version to be published.
Corresponding author
Ethics declarations
None of the investigators has personal or financial interests in the conduct or the outcomes of the project. IIKS has received speaker grants from Roche Diabetes Care and Rubin Medical. SS has served on the continuous glucose monitoring advisory board of Roche Diabetes Care. JJH has consulted for Merck Sharp & Dome, Novo Nordisk and Roche. TRC works for Novo Nordisk A/S and own shares in Novo Nordisk A/S and Zealand Pharma A/S. KN serves as adviser to Medtronic, Abbott and Novo Nordisk A/S, owns shares in Novo Nordisk A/S, has received research grants from Novo Nordisk A/S and Roche Diabetes Care and has received fees for speaking from Medtronic, Roche Diabetes Care, Rubin Medical, Sanofi, Novo Nordisk A/S, Zealand Pharma and Bayer. AR declares that there is no duality of interest associated with his contribution to this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM Fig.1
(PDF 119 kb)
Rights and permissions
About this article
Cite this article
Steineck, I.I.K., Ranjan, A., Schmidt, S. et al. Preserved glucose response to low-dose glucagon after exercise in insulin-pump-treated individuals with type 1 diabetes: a randomised crossover study. Diabetologia 62, 582–592 (2019). https://doi.org/10.1007/s00125-018-4807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4807-8