Abstract
Aims/hypothesis
Diabetes has been shown to be a risk factor for some cancers. Whether diabetes confers the same excess risk of cancer, overall and by site, in women and men is unknown.
Methods
A systematic search was performed in PubMed for cohort studies published up to December 2016. Selected studies reported sex-specific relative risk (RR) estimates for the association between diabetes and cancer adjusted at least for age in both sexes. Random-effects meta-analyses with inverse-variance weighting were used to obtain pooled sex-specific RRs and women-to-men ratios of RRs (RRRs) for all-site and site-specific cancers.
Results
Data on all-site cancer events (incident or fatal only) were available from 121 cohorts (19,239,302 individuals; 1,082,592 events). The pooled adjusted RR for all-site cancer associated with diabetes was 1.27 (95% CI 1.21, 1.32) in women and 1.19 (1.13, 1.25) in men. Women with diabetes had ~6% greater risk compared with men with diabetes (the pooled RRR was 1.06, 95% CI 1.03, 1.09). Corresponding pooled RRRs were 1.10 (1.07, 1.13) for all-site cancer incidence and 1.03 (0.99, 1.06) for all-site cancer mortality. Diabetes also conferred a significantly greater RR in women than men for oral, stomach and kidney cancer, and for leukaemia, but a lower RR for liver cancer.
Conclusions/interpretation
Diabetes is a risk factor for all-site cancer for both women and men, but the excess risk of cancer associated with diabetes is slightly greater for women than men. The direction and magnitude of sex differences varies by location of the cancer.
Similar content being viewed by others

Introduction
Cancer is the second leading causes of death in the world [1]. In 2015, there were 17.5 million incident cancer cases and 8.7 million cancer deaths globally, and it is estimated that one in four women and one in three men develop cancer during their lifetime [2]. The incidence of cancer is expected to increase in the next decades, emphasising the importance of efficient prevention and treatment of cancer worldwide.
The prevalence of diabetes has also grown rapidly. In 2015, one in 11 adults (415 million) were reported to have diabetes, five million deaths were attributed to diabetes, and 12% of global health expenditure was spent on diabetes and its complications [3]. Diabetes has been associated with the risk of all-site and some site-specific cancers in several systematic reviews and meta-analyses [4,5,6,7,8,9,10,11,12,13]. However, only a minority of these associations are based on robust supporting evidence without question of significant bias [14]. To date, there has been no systematic overview of the evidence available on sex differences in the association between diabetes and cancer. We have previously published compelling evidence that women with diabetes are at an increased risk of stroke [15], coronary heart disease [16] and dementia [17] compared with their male peers. We now question whether this is also true for cancer. In this study, we conducted the most comprehensive systematic review and meta-analysis, to date, to estimate the relative effect of diabetes on the risk of cancer in women compared with men.
Methods
Search strategy and selection criteria
A systematic search was performed in PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) on 23 December 2016 using a combined text word and medical subject heading search strategy (electronic supplementary material [ESM] Table 1). The reference lists of identified reports were also checked for other potentially relevant studies.
Observational cohort studies in general populations were included if they had provided relative risks (RRs), or equivalents, for the association between diabetes and cancer in both women and men. Studies were excluded if they had not adjusted at least for age or did not provide information about the variability around the point estimate, or if they only had data for one sex. In case of duplicate reports from the same study, the study providing the longest follow-up or the highest number of cases was included. We also used individual participant data from the Asia Pacific Cohort Studies Collaboration (APCSC) [18], treated as two separate combinations of data from cohorts in Asia and cohorts from Australia or New Zealand, as in our previous work [15, 16]. One author (TO) did the search and extracted the data. Uncertainties regarding the inclusion or exclusion of articles and data extraction were discussed by all authors and resolved by mutual consent. The meta-analysis was done in accordance with the PRISMA criteria [19].
Data extraction and statistical analysis
The primary endpoint was all-site cancer events (incident or, if this was all that was presented, mortal only). The secondary endpoints were all-site cancer incidence (i.e. omitting studies that only reported mortality), all-site cancer mortality and, for those cancers that could present in both sexes, site-specific cancer events, site-specific incidence and site-specific mortality. In sensitivity analysis we also compared all-site cancer incidence and mortality when restricting to the studies that reported both.
The primary metrics were the pooled adjusted RRs and the women-to-men ratios of RRs (RRRs) for individuals with diabetes vs those without diabetes. For each study, we extracted the sex-specific RRs and 95% CIs for individuals with diabetes vs those without diabetes, from which we estimated the RRRs and 95% CIs. To include the largest set of individuals and cancer endpoints, studies that reported either age-adjusted or multiple-adjusted (maximum-available-adjusted, i.e. the maximum set of adjustments available for each study) results were included in our primary analyses. In pooling multiple-adjusted results, the set of adjustments made were allowed to vary by study, but had to include at least one other risk factor for cancer, in addition to age [15, 16]. We obtained pooled estimates of sex-specific RRs across studies using random-effects meta-analyses applied on the loge scale. Individual studies were weighted according to the inverse variance of loge RRs. The same method was used to pool the RRRs.
The I2 statistic was used to estimate the percentage of variability across studies due to between-study heterogeneity and the Q test was used to assess whether there was a significant lack of homogeneity. The possibility of publication bias was explored using funnel plots and Egger’s and Begg’s tests. Random-effects meta-regression analyses were used to test for differences between pre-assigned subgroups: study region (Asia or Non-Asia), year of baseline study (pre-1985 or 1986 onwards, and also examined as a continuous variable), ascertainment of diabetes (self-reported only or others), type of diabetes (type 1 or type 2, where studies which did not differentiate type were classified as type 2), level of adjustment (age-adjusted or multiple-adjusted), and study quality (the Newcastle–Ottawa Scale [20] [ESM Table 2], ≥7 or <7 points, and also examined as a continuous variable). Post hoc, we also considered absolute risk difference, examined as a categorical and continuous variable) (ESM Table 3). A p value of below 0.05 was considered to be statistically significant in analyses for the primary analyses, i.e. all-site cancer. As many statistical tests were envisaged, a p value of below 0.01 was taken to denote significance for site-specific cancers. All analyses were performed using Stata software (release 13; StataCorp, College Station, TX, USA).
Results
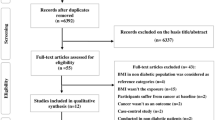
Of the 6371 articles identified through the systematic search, 371 articles qualified for full-text evaluation, and 107 articles provided summary data on the association between diabetes and the risk of cancer for both sexes [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127]. In addition, 36 cohorts with individual participant data from the APCSC were included (Fig. 1).
Characteristics of the studies that reported the association between diabetes and all-site cancer incidence or mortality are shown in Table 1 and ESM Table 4. Data on all-site cancer were available from 47 studies, involving 121 cohorts, 19,239,302 individuals (not counting one study [25] that did not state the total number of participants), and 1,082,592 events (not counting one study [65] that did not state the total number of cancer events).
The maximum-available-adjusted pooled sex-specific RR estimates for combined fatal and non-fatal cancer associated with diabetes were 1.27 (95% CI 1.21, 1.32, p < 0.001) for women and 1.19 (1.13, 1.25, p < 0.001) for men (Fig. 2). The pooled women-to-men RRR was 1.06 (1.03, 1.09, p < 0.001, Fig. 3). The I2 statistic for heterogeneity between studies was 66.7%, with no evidence of publication bias (Egger’s test p = 0.13, Begg’s test p = 0.16, ESM Fig. 1). The corresponding RRR was 1.06 (1.02, 1.11, p = 0.005) for type 1 diabetes and 1.06 (1.03, 1.09, p < 0.001) for type 2 diabetes, without evidence of significant heterogeneity by type of diabetes (p for interaction = 0.88, Fig. 4). Exclusion of 22 studies that provided only age-adjusted results had no appreciable effect on the pooled RR estimates (multiple-adjusted pooled RR in women 1.25 [1.17, 1.34], p < 0.001, RR in men 1.20 [1.11, 1.29], p < 0.001, RRR 1.06 [1.03, 1.10], p < 0.001, I2 = 48.9%) (ESM Figs 2 and 3).
Maximum-available-adjusted RR for all-site cancer, comparing individuals with diabetes with those without diabetes by sex: (a) women; and (b) men. ANZ, Australia and New Zealand; ARIC, Atherosclerosis Risk in Communities; BRFSS, Behavioral Risk Factor Surveillance System; CLUE II, Give Us a Clue to Cancer and Heart Disease; CPS II, Cancer Prevention Study II; DECODE, Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe; DERI, Diabetes Epidemiology Research International; DRT, Diabetes Registry Tyrol; 2001 ENTRED study, 2001–2006 National representative sample of people with diabetes study; HSE, Health Survey for England; MHS, Maccabi Healthcare Services; NDSS, National Diabetes Services Scheme; NIH-AARP, National Institutes of Health-American Association of Retired Persons; NHIS-NSC, Korean National Health Insurance Service-National Sample Cohort; SHeS, Scottish Health Survey; VHM&PP, The Vorarlberg Health Monitoring and Promotion Programme
Maximum-available-adjusted women-to-men RRR for all-site cancer, comparing individuals with diabetes with those without diabetes. For definition of study acronyms, please refer to Fig. 2 legend. aThe BRFSS did not report the total number of cancer events
Subgroup analyses of women-to-men RRR for all-site cancer, comparing individuals with diabetes with those without diabetes. aSix studies were excluded because the baseline year bridged over 1985 (i.e. included both pre-1985 and 1986 onwards). bResults using multiple adjustment were used when available and age-adjusted otherwise, as in Fig. 3. cTen studies were excluded because absolute risks for men and women were unavailable
The pooled RRR did not vary substantially by study region (p = 0.45), year of baseline study (p = 0.54 for categorical analysis, p = 0.18 for continuous analysis), ascertainment of diabetes (p = 0.72), level of adjustment (p = 0.70), quality of study (p = 0.09 for categorical analysis) or absolute risk difference between men and women (p = 0.82 for categorical analysis, p = 0.99 for continuous analysis), with the exception of continuous analysis for quality of study, p = 0.01) (Fig. 4 and ESM Fig. 4).
Secondary analyses of incidence (fatal or not) and mortality alone for all-site cancer are described in the ESM. The pooled women-to-men RRR for incidence was 1.10 (1.07, 1.13, p < 0.001) (ESM Fig. 5) and for mortality was 1.03 (0.99, 1.06, p = 0.16) (ESM Fig. 6).
In sensitivity analysis using only those studies which provided the RRs for both incidence and mortality, the pooled maximum-available-adjusted RRR was 1.12 (1.06, 1.17, p < 0.001) for all-site cancer incidence, and 1.10 (1.00, 1.21, p = 0.04) for all-site cancer mortality (ESM Fig. 7).
Data on site-specific cancer were available for 50 sites (50 sites for incidence and 29 sites for mortality) (https://www.georgeinstitute.org/sites/default/files/esm-table.pdf). Diabetes was associated with an increased risk of cancer in 43 sites in women and 42 sites in men, with a statistically significant increase (p < 0.01) in risk for those with diabetes in 20 sites in women and 18 sites in men (ESM Fig. 8). The pooled maximum-available-adjusted RRR was statistically significantly higher in women than men for kidney (1.11 [99% CI 1.04, 1.18], p < 0.001), oral (1.13 [1.00, 1.28], p = 0.009), stomach cancer (1.14 [1.07, 1.22], p < 0.001) and leukaemia (1.15 [1.02, 1.28], p = 0.002), whereas it was statistically significantly lower for liver cancer (0.88 [0.79, 0.99], p = 0.005) (Fig. 5). Separate results for incidence and mortality by site of cancer are described in the ESM (ESM Figs 5, 6, 9–24).
Discussion
This systematic review, with meta-analysis, of 121 cohorts including more than 19 million individuals and over one million all-site cancer events, demonstrated that diabetes was associated with a 6% higher excess risk of all-site cancer in women than men. Diabetes was associated with several site-specific cancers and conferred a significantly greater excess risk in women than men for oral, stomach and kidney cancer and for leukaemia, but a lower excess risk for liver cancer. The findings were broadly consistent for incident and fatal cancers and across a wide range of prespecified subgroups.
Our findings are in agreement with a previous meta-analysis, which found that the risk of all-site cancer incidence and mortality was significantly increased in both sexes [4]. However, this previous meta-analysis was about a tenth of the size of the current study, and included single-sex studies, and therefore was not able to reliably quantify sex differences as they could have been explained by differences in methods, confounders adjusted for, and the background risks between studies of women and men alone.
As we found some evidence to suggest that the women-to-men RRRs tended to be smaller in studies of lower quality (Fig. 4 and ESM Fig. 4), our results may underestimate any true sex difference. A significant degree of heterogeneity was also observed between studies conducted in and outside Asia with regards to all-site cancer mortality (ESM Fig. 19). However, we did not find heterogeneity between regions for our primary outcome, nor for the other secondary outcomes (all-site cancer incidence), and thus we speculate that this may be a chance finding consequent to the high number of statistical tests conducted.
Although we found a slightly higher women-to-men RRR for cancer incidence than cancer mortality, the finding may be explained by chance differences between the included studies, as almost identical pooled RRR estimates were obtained in the sensitivity analysis restricted to five studies which provided the sex-specific RRs for both incidence and mortality from the same study.
With regard to cancer at specific sites, previous meta-analyses have yielded inconsistent results of increased (stomach [5], lung [6], kidney [7]), similar (oesophagus [8], colorectum [9], pancreas [10], bladder [11], thyroid [12]) or decreased (liver [13]) excess risk of cancer associated with diabetes in women compared with men. However, unlike our methods, these analyses included single-sex studies as well as studies among both women and men.
There are several possible explanations for the excess risk of cancer conferred by diabetes in women than men. One possible mechanism is poor glycaemic control in women with diabetes compared with men with diabetes [128, 129]. Hyperglycaemia may have carcinogenic effects by causing DNA damage [130], which could result from increased oxidative stress due to hyperglycaemia [130] or from hyperglycaemia itself [131]. Historically, women were likely to be undertreated or receive less intensive care compared with men [128, 132]. Further, a recent study showed that adherence to glucose-lowering medication was lower in women than men [133]. As such, the carcinogenic effects of hyperglycaemia may be enhanced in women and subsequently lead to an increased cancer risk compared with men. Alternatively, cumulative exposure to insulin resistance and subsequent hyperinsulinaemia may be longer in women compared with men. The average duration of impaired glucose tolerance or impaired fasting glucose has been found to be more than 2 years longer in women than men [134], suggesting that women may have more exposure to, often untreated, hyperinsulinaemia in the prediabetic state. Hyperinsulinaemia promotes cancer cell proliferation by stimulating the insulin receptor directly and insulin-like growth factor-1 indirectly [135]. Another factor that may, to some extent, explain the smaller RR for incidence of all-site cancer in men compared with women is the apparent protective effect of diabetes on prostate cancer in men with diabetes [136]. Sex-specific cancers or site-specific cancers in which diabetes conferred greater or lower excess risk in women than men may also account for the association, although the degree of contribution cannot be determined from our analyses. In addition to sex difference for all-site cancer, we found also that diabetes conferred a significantly greater RR in women than men for oral, stomach and kidney cancer and for leukaemia, but a lower RR for liver cancer. The underlying mechanisms for sex differences in each specific association are not clear. However, unmeasured confounding factors specific to each site, such as Helicobacter pylori infection for stomach cancer [137] and hepatitis virus infection for liver cancer [138], might be involved. However, the literature around mechanisms underpinning the sex differences in site-specific cancers is scant and further studies are required to confirm and clarify these sex differences in site-specific associations. Finally, the studies in our analyses were not adjusted for female-specific factors including pregnancy, menopausal status and use of hormone replacement therapy that have also been associated with diabetes [139] and cancer [140].
We quantified sex differences based on RRs rather than risk differences. This might introduce a statistical artefact, in which the generally higher absolute risk for cancer in men, and the same risk difference subsequent to diabetes in each sex, would translate to a greater relative risk in women than men. However, this would require that risks of cancers associated with diabetes are additive rather than multiplicative, which is not generally considered to be the case in epidemiology. Indeed, RRs are much more commonly reported than risk differences in both epidemiological studies and clinical trials. Also, unlike risk differences, RRs are typically fairly stable across populations with different background risks, which make them suitable for summarisation of effects in meta-analyses. Furthermore, our previous meta-analyses on risk factors for cardiovascular diseases demonstrated that detection of a female disadvantage in RRs is not inevitable when men have higher absolute risk [141, 142]. We thus believe that the use of RRs in the present analyses is both practical and justifiable.
The strengths of this meta-analysis are its size and the inclusion of studies on the sex-specific effects of diabetes on all-site cancer and 50 site-specific cancers, which enabled us to conduct the most comprehensive analyses to date on the sex-specific effects of diabetes on cancer risk. To limit the risk of bias, we only included cohort studies that were conducted in men and women and had adjusted for at least age. Limitations of this study are inherent to the use of published data and the heterogeneity between studies in ascertainment of diabetes, study design and duration, endpoint definition and degree of adjustment for confounders. Nevertheless, a range of subgroup analysis provided broadly consistent results. However, as we compared women and men from within the same study, any effect of differences in methods between studies is likely to have affected women and men similarly. We therefore assume that the sex comparisons reported in this analysis are still valid. Second, the lack of data on duration of diabetes and the degree of glycaemic control precluded more detailed analyses on the effect of diabetes on the risk of cancer. Third, as this meta-analysis largely used published data, endpoint definition varied across the studies. Fourth, in analysis of all-site cancer, the women-to-men RRRs depend not only on the strengths of the RRRs of site-specific cancers (as illustrated by Fig. 5), but also on the relative incidence of site-specific cancers, which varies considerably between populations. This is likely to be a key factor in the high between-study heterogeneity we show in Fig. 3. Finally, studies generally did not adjust for obstetric and gynaecological history and unmeasured confounding is likely in the current estimates. However, confounding is likely to have been non-differentially distributed between women and men from the same study and we therefore assume that it had only a negligible effect on the reported associations.
In conclusion, diabetes is a risk factor for all-site cancer in both sexes, with a stronger effect in women than men. Sex differences varied across the location of the cancer, heightening the importance of a sex-specific approach to quantification of the role of diabetes in cancer research, prevention and treatment. Further studies are needed to clarify the mechanisms underlying the sex differences in the diabetes–cancer association.
Data availability
The datasets generated during and/or analysed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- APCSC:
-
Asia Pacific Cohort Studies Collaboration
- RRR:
-
Ratio of RR
References
GBD 2015 Mortality and Causes of Death Collaborators (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1459–1544
Fitzmaurice C, Allen C, Barber RM et al (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 3:524–548
International Diabetes Federation (2015) IDF Diabetes Atlas, 7th edn. IDF, Brussels. Available from https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventhedition.html. Accessed 12 May 2018
Noto H, Tsujimoto T, Sasazuki T, Noda M (2011) Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract 17:616–628
Ge Z, Ben Q, Qian J, Wang Y, Li Y (2011) Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol 23:1127–1135
Lee JY, Jeon I, Lee JM, Yoon JM, Park SM (2013) Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer 49:2411–2423
Bao C, Yang X, Xu W et al (2013) Diabetes mellitus and incidence and mortality of kidney cancer: a meta-analysis. J Diabetes Complicat 27:357–364
Huang W, Ren H, Ben Q, Cai Q, Zhu W, Li Z (2012) Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control 23:263–272
Kramer HU, Schottker B, Raum E, Brenner H (2012) Type 2 diabetes mellitus and colorectal cancer: meta-analysis on sex-specific differences. Eur J Cancer 48:1269–1282
Ben Q, Xu M, Ning X et al (2011) Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer 47:1928–1937
Zhu Z, Wang X, Shen Z, Lu Y, Zhong S, Xu C (2013) Risk of bladder cancer in patients with diabetes mellitus: an updated meta-analysis of 36 observational studies. BMC Cancer 13:310
Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M (2013) Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol 28:945–958
Wang Y, Wang B, Yan S et al (2016) Type 2 diabetes and gender differences in liver cancer by considering different confounding factors: a meta-analysis of cohort studies. Ann Epidemiol 26:764–772
Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350:g7607
Peters SA, Huxley RR, Woodward M (2014) Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 383:1973–1980
Peters SA, Huxley RR, Woodward M (2014) Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 57:1542–1551
Chatterjee S, Peters SA, Woodward M et al (2016) Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39:300–307
Lam EK, Batty GD, Huxley RR et al (2011) Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol 22:730–738
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Wells G, Shea B, O’Connell D et al. (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 17 Jun 2017
Ragozzino M, Melton IIILJ, Chu CP, Palumbo PJ (1982) Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis 35:13–19
Sasazuki S, Charvat H, Hara A et al (2013) Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci 104:1499–1507
Gini A, Bidoli E, Zanier L et al (2016) Cancer among patients with type 2 diabetes mellitus: a population-based cohort study in northeastern Italy. Cancer Epidemiol 41:80–87
Berger SM, Gislason G, Moore LL et al (2016) Associations between metabolic disorders and risk of cancer in Danish men and women—a nationwide cohort study. BMC Cancer 16:133
Carstensen B, Read SH, Friis S et al (2016) Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia 59:980–988
Hense HW, Kajuter H, Wellmann J, Batzler WU (2011) Cancer incidence in type 2 diabetes patients—first results from a feasibility study of the D2C cohort. Diabetol Metab Syndr 3:15
Rapp K, Schroeder J, Klenk J et al (2006) Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia 49:945–952
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM (2005) Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293:194–202
Wang M, Hu RY, Wu HB et al (2015) Cancer risk among patients with type 2 diabetes mellitus: a population-based prospective study in China. Sci Rep 5:11503
Hsu PC, Lin WH, Kuo TH, Lee HM, Kuo C, Li CY (2015) A population-based cohort study of all-cause and site-specific cancer incidence among patients with type 1 diabetes mellitus in Taiwan. J Epidemiol 25:567–573
Adami HO, McLaughlin J, Ekbom A et al (1991) Cancer risk in patients with diabetes mellitus. Cancer Causes Control 2:307–314
Dankner R, Boffetta P, Balicer RD et al (2016) Time-dependent risk of cancer after a diabetes diagnosis in a cohort of 2.3 million adults. Am J Epidemiol 183:1098–1106
Lai GY, Park Y, Hartge P, Hollenbeck AR, Freedman ND (2013) The association between self-reported diabetes and cancer incidence in the NIH-AARP Diet and Health Study. J Clin Endocrinol Metab 98:E497–E502
Xu HL, Fang H, Xu WH et al (2015) Cancer incidence in patients with type 2 diabetes mellitus: a population-based cohort study in Shanghai. BMC Cancer 15:852
Oberaigner W, Ebenbichler C, Oberaigner K, Juchum M, Schonherr HR, Lechleitner M (2014) Increased cancer incidence risk in type 2 diabetes mellitus: results from a cohort study in Tyrol/Austria. BMC Public Health 14:1058
Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ (2015) Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 38:264–270
Walker JJ, Brewster DH, Colhoun HM et al (2013) Type 2 diabetes, socioeconomic status and risk of cancer in Scotland 2001-2007. Diabetologia 56:1712–1715
Chodick G, Heymann AD, Rosenmann L et al (2010) Diabetes and risk of incident cancer: a large population-based cohort study in Israel. Cancer Causes Control 21:879–887
Yeh HC, Platz EA, Wang NY, Visvanathan K, Helzlsouer KJ, Brancati FL (2012) A prospective study of the associations between treated diabetes and cancer outcomes. Diabetes Care 35:113–118
Zhang PH, Chen ZW, Lv D et al (2012) Increased risk of cancer in patients with type 2 diabetes mellitus: a retrospective cohort study in China. BMC Public Health 12:567
Stattin P, Bjor O, Ferrari P et al (2007) Prospective study of hyperglycemia and cancer risk. Diabetes Care 30:561–567
Joshu CE, Prizment AE, Dluzniewski PJ et al (2012) Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990-2006. Int J Cancer 131:1667–1677
Wideroff L, Gridley G, Mellemkjaer L et al (1997) Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 89:1360–1365
Bancks MP, Odegaard AO, Pankow JS et al (2014) Glycated hemoglobin and all-cause and cause-specific mortality in Singaporean Chinese without diagnosed diabetes: the Singapore Chinese Health Study. Diabetes Care 37:3180–3187
Guzder RN, Gatling W, Mullee MA, Byrne CD (2007) Early mortality from the time of diagnosis of type 2 diabetes: a 5-year prospective cohort study with a local age- and sex-matched comparison cohort. Diabet Med 24:1164–1167
Morimoto A, Onda Y, Nishimura R, Sano H, Utsunomiya K, Tajima N (2013) Cause-specific mortality trends in a nationwide population-based cohort of childhood-onset type 1 diabetes in Japan during 35 years of follow-up: the DERI Mortality Study. Diabetologia 56:2171–2175
Swerdlow AJ, Laing SP, Dos Santos Silva I et al (2004) Mortality of South Asian patients with insulin-treated diabetes mellitus in the United Kingdom: a cohort study. Diabet Med 21:845–851
Kato M, Noda M, Mizoue T et al (2015) Diagnosed diabetes and premature death among middle-aged Japanese: results from a large-scale population-based cohort study in Japan (JPHC study). BMJ Open 5:e007736
Baena-Diez JM, Penafiel J, Subirana I et al (2016) Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care 39:1987–1995
Kang YM, Kim YJ, Park JY, Lee WJ, Jung CH (2016) Mortality and causes of death in a national sample of type 2 diabetic patients in Korea from 2002 to 2013. Cardiovasc Diabetol 15:131
Zhou XH, Qiao Q, Zethelius B et al (2010) Diabetes, prediabetes and cancer mortality. Diabetologia 53:1867–1876
Tseng CH (2004) Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care 27:1605–1609
Gnavi R, Petrelli A, Demaria M, Spadea T, Carta Q, Costa G (2004) Mortality and educational level among diabetic and non-diabetic population in the Turin Longitudinal Study: a 9-year follow-up. Int J Epidemiol 33:864–871
Hirakawa Y, Ninomiya T, Mukai N et al (2012) Association between glucose tolerance level and cancer death in a general Japanese population: the Hisayama Study. Am J Epidemiol 176:856–864
Forssas E, Sund R, Manderbacka K, Arffman M, Ilanne-Parikka P, Keskimaki I (2013) Increased cancer mortality in diabetic people treated with insulin: a register-based follow-up study. BMC Health Serv Res 13:267
Fedeli U, Zoppini G, Gennaro N, Saugo M (2014) Diabetes and cancer mortality: a multifaceted association. Diabetes Res Clin Pract 106:e86–e89
Gordon-Dseagu VL, Shelton N, Mindell J (2014) Diabetes mellitus and mortality from all-causes, cancer, cardiovascular and respiratory disease: evidence from the Health Survey for England and Scottish Health Survey cohorts. J Diabetes Complicat 28:791–797
Shen C, Schooling CM, Chan WM, Lee SY, Leung GM, Lam TH (2014) Self-reported diabetes and mortality in a prospective Chinese elderly cohort study in Hong Kong. Prev Med 64:20–26
Weiderpass E, Gridley G, Nyren O, Pennello G, Landstrom AS, Ekbom A (2001) Cause-specific mortality in a cohort of patients with diabetes mellitus: a population-based study in Sweden. J Clin Epidemiol 54:802–809
Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM (2012) Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care 35:1835–1844
Verlato G, Zoppini G, Bonora E, Muggeo M (2003) Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care 26:1047–1051
Sievers ML, Nelson RG, Knowler WC, Bennett PH (1992) Impact of NIDDM on mortality and causes of death in Pima Indians. Diabetes Care 15:1541–1549
Romon I, Rey G, Mandereau-Bruno L et al (2014) The excess mortality related to cardiovascular diseases and cancer among adults pharmacologically treated for diabetes—the 2001-2006 ENTRED cohort. Diabet Med 31:946–953
Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ (2010) Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 59:3216–3222
Tierney EF, Geiss LS, Engelgau MM et al (2001) Population-based estimates of mortality associated with diabetes: use of a death certificate check box in North Dakota. Am J Public Health 91:84–92
Wong JS, Pearson DW, Murchison LE, Williams MJ, Narayan V (1991) Mortality in diabetes mellitus: experience of a geographically defined population. Diabet Med 8:135–139
Bruno G, Merletti F, Boffetta P et al (1999) Impact of glycaemic control, hypertension and insulin treatment on general and cause-specific mortality: an Italian population-based cohort of type II (non-insulin-dependent) diabetes mellitus. Diabetologia 42:297–301
Shaw JE, Hodge AM, de Courten M, Chitson P, Zimmet PZ (1999) Isolated post-challenge hyperglycaemia confirmed as a risk factor for mortality. Diabetologia 42:1050–1054
Moss SE, Klein R, Klein BE (1991) Cause-specific mortality in a population-based study of diabetes. Am J Public Health 81:1158–1162
Oba S, Nagata C, Nakamura K, Takatsuka N, Shimizu H (2008) Self-reported diabetes mellitus and risk of mortality from all causes, cardiovascular disease, and cancer in Takayama: a population-based prospective cohort study in Japan. J Epidemiol 18:197–203
Levine W, Dyer AR, Shekelle RB, Schoenberger JA, Stamler J (1990) Post-load plasma glucose and cancer mortality in middle-aged men and women. 12-year follow-up findings of the Chicago Heart Association Detection Project in Industry. Am J Epidemiol 131:254–262
Idilbi NM, Barchana M, Milman U, Carel RS (2013) Incidence of cancer among diabetic and non-diabetic adult Israeli Arabs. Isr Med Assoc J 15:342–347
Hippisley-Cox J, Coupland C (2015) Development and validation of risk prediction algorithms to estimate future risk of common cancers in men and women: prospective cohort study. BMJ Open 5:e007825
Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K (2010) Cancer risk among patients hospitalized for type 1 diabetes mellitus: a population-based cohort study in Sweden. Diabet Med 27:791–797
Lin CC, Chiang JH, Li CI et al (2014) Cancer risks among patients with type 2 diabetes: a 10-year follow-up study of a nationwide population-based cohort in Taiwan. BMC Cancer 14:381
Xu HL, Tan YT, Epplein M et al (2015) Population-based cohort studies of type 2 diabetes and stomach cancer risk in Chinese men and women. Cancer Sci 106:294–298
Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N (2004) Preliminary communication: glycated hemoglobin, diabetes, and incident colorectal cancer in men and women: a prospective analysis from the European prospective investigation into cancer-Norfolk study. Cancer Epidemiol Biomark Prev 13:915–919
Limburg PJ, Vierkant RA, Fredericksen ZS et al (2006) Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 101:1872–1879
Schoen RE, Tangen CM, Kuller LH et al (1999) Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 91:1147–1154
Campbell PT, Deka A, Jacobs EJ et al (2010) Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 139:1138–1146
Goto A, Noda M, Sawada N et al (2016) High hemoglobin A1c levels within the non-diabetic range are associated with the risk of all cancers. Int J Cancer 138:1741–1753
Will JC, Galuska DA, Vinicor F, Calle EE (1998) Colorectal cancer: another complication of diabetes mellitus? Am J Epidemiol 147:816–825
Seow A, Yuan JM, Koh WP, Lee HP, Yu MC (2006) Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst 98:135–138
Magliano DJ, Davis WA, Shaw JE, Bruce DG, Davis TM (2012) Incidence and predictors of all-cause and site-specific cancer in type 2 diabetes: the Fremantle Diabetes Study. Eur J Endocrinol 167:589–599
Jarvandi S, Davidson NO, Schootman M (2013) Increased risk of colorectal cancer in type 2 diabetes is independent of diet quality. PLoS One 8:e74616
Sikdar KC, Walsh SJ, Roche M, Jiang Y, Syrowatka A, Collins KD (2013) Diabetes and sex-specific colorectal cancer risks in Newfoundland and Labrador: a population-based retrospective cohort study. Can J Public Health 104:e101–e107
He J, Stram DO, Kolonel LN, Henderson BE, Le Marchand L, Haiman CA (2010) The association of diabetes with colorectal cancer risk: the Multiethnic Cohort. Br J Cancer 103:120–126
de Kort S, Simons CC, van den Brandt PA et al (2016) Diabetes mellitus type 2 and subsite-specific colorectal cancer risk in men and women: results from the Netherlands Cohort Study on diet and cancer. Eur J Gastroenterol Hepatol 28:896–903
Nilsen TI, Vatten LJ (2001) Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer 84:417–422
Koskinen SV, Reunanen AR, Martelin TP, Valkonen T (1998) Mortality in a large population-based cohort of patients with drug-treated diabetes mellitus. Am J Public Health 88:765–770
Tan C, Mori M, Adachi Y et al (2016) Diabetes mellitus and risk of colorectal Cancer mortality in Japan: the Japan Collaborative Cohort Study. Asian Pac J Cancer Prev 17:4681–4688
Ren X, Zhang X, Zhang X et al (2009) Type 2 diabetes mellitus associated with increased risk for colorectal cancer: evidence from an international ecological study and population-based risk analysis in China. Public Health 123:540–544
Chen HF, Chen P, Su YH, Su HF, Li CY (2012) Age- and sex-specific risks of colorectal cancers in diabetic patients. Tohoku J Exp Med 226:259–265
Weiderpass E, Gridley G, Nyren O, Ekbom A, Persson I, Adami HO (1997) Diabetes mellitus and risk of large bowel cancer. J Natl Cancer Inst 89:660–661
Onitilo AA, Berg RL, Engel JM et al (2013) Increased risk of colon cancer in men in the pre-diabetes phase. PLoS One 8:e70426
Adami HO, Chow WH, Nyren O et al (1996) Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst 88:1472–1477
Campbell PT, Newton CC, Freedman ND et al (2016) Body mass index, waist circumference, diabetes, and risk of liver Cancer for U.S. adults. Cancer Res 76:6076–6083
Yang WS, Shu XO, Gao J et al (2013) Prospective evaluation of type 2 diabetes mellitus on the risk of primary liver cancer in Chinese men and women. Ann Oncol 24:1679–1685
Koh WP, Wang R, Jin A, Yu MC, Yuan JM (2013) Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer 108:1182–1188
Wild SH, Morling JR, McAllister DA et al (2016) Type 2 diabetes and risk of hospital admission or death for chronic liver diseases. J Hepatol 64:1358–1364
Fujino Y, Mizoue T, Tokui N, Yoshimura T (2001) Prospective study of diabetes mellitus and liver cancer in Japan. Diabetes Metab Res Rev 17:374–379
Shibata A, Ogimoto I, Kurozawa Y et al (2003) Past medical history and risk of death due to hepatocellular carcinoma, univariate analysis of JACC study data. Kurume Med J 50:109–119
Chiang CH, Lee LT, Hung SH et al (2014) Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology 59:2207–2215
Chen HF, Chen P, Li CY (2010) Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology 52:155–163
Yagyu K, Lin Y, Obata Y et al (2004) Bowel movement frequency, medical history and the risk of gallbladder cancer death: a cohort study in Japan. Cancer Sci 95:674–678
Tsai MS, Lee PH, Lin CL, Peng CL, Kao CH (2015) Type II diabetes mellitus is associated with a reduced risk of cholangiocarcinoma in patients with biliary tract diseases. Int J Cancer 136:2409–2417
Larsson SC, Permert J, Hakansson N, Naslund I, Bergkvist L, Wolk A (2005) Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer 93:1310–1315
Nilsen TI, Vatten LJ (2000) A prospective study of lifestyle factors and the risk of pancreatic cancer in Nord-Trondelag, Norway. Cancer Causes Control 11:645–652
Chow WH, Gridley G, Nyren O et al (1995) Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. J Natl Cancer Inst 87:930–931
Lin Y, Tamakoshi A, Kawamura T et al (2002) Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. Int J Cancer 99:742–746
Hall GC, Roberts CM, Boulis M, Mo J, MacRae KD (2005) Diabetes and the risk of lung cancer. Diabetes Care 28:590–594
Yang WS, Yang Y, Yang G et al (2014) Pre-existing type 2 diabetes and risk of lung cancer: a report from two prospective cohort studies of 133 024 Chinese adults in urban Shanghai. BMJ Open 4:e004875
Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE (2007) Risk factors for renal cell cancer: the Multiethnic Cohort. Am J Epidemiol 166:932–940
Lindblad P, Chow WH, Chan J et al (1999) The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 42:107–112
Washio M, Mori M, Khan M et al (2007) Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int J Urol 14:393–397
Goossens ME, Zeegers MP, Bazelier MT, De Bruin ML, Buntinx F, de Vries F (2015) Risk of bladder cancer in patients with diabetes: a retrospective cohort study. BMJ Open 5:e007470
Newton CC, Gapstur SM, Campbell PT, Jacobs EJ (2013) Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 132:2186–2191
Woolcott CG, Maskarinec G, Haiman CA, Henderson BE, Kolonel LN (2011) Diabetes and urothelial cancer risk: the Multiethnic Cohort study. Cancer Epidemiol 35:551–554
Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S (2006) Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 166:1871–1877
Khan M, Mori M, Fujino Y et al (2006) Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev 7:253–259
Khan AE, Gallo V, Linseisen J et al (2008) Diabetes and the risk of non-Hodgkin’s lymphoma and multiple myeloma in the European Prospective Investigation into Cancer and Nutrition. Haematologica 93:842–850
Yang WS, Li HL, Xu HL et al (2016) Type 2 diabetes and the risk of non-Hodgkin’s lymphoma: a report from two population-based cohort studies in China. Eur J Cancer Prev 25:149–154
Erber E, Lim U, Maskarinec G, Kolonel LN (2009) Common immune-related risk factors and incident non-Hodgkin lymphoma: the Multiethnic Cohort. Int J Cancer 125:1440–1445
Weiderpass E, Gridley G, Ekbom A, Nyren O, Hjalgrim H, Adami HO (1997) Medical history risk factors for non-Hodgkin’s lymphoma in older women. J Natl Cancer Inst 89:816–817
Weiderpass E, Gridley G, Persson I, Nyren O, Ekbom A, Adami HO (1997) Risk of endometrial and breast cancer in patients with diabetes mellitus. Int J Cancer 71:360–363
Kitahara CM, Platz EA, Beane Freeman LE et al (2012) Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control 23:463–471
Hemminki K, Forsti A, Sundquist K, Li X (2016) Cancer of unknown primary is associated with diabetes. Eur J Cancer Prev 25:246–251
Kautzky-Willer A, Kamyar MR, Gerhat D et al (2010) Sex-specific differences in metabolic control, cardiovascular risk, and interventions in patients with type 2 diabetes mellitus. Gend Med 7:571–583
Petitti DB, Klingensmith GJ, Bell RA et al (2009) Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 155:668–672.e661-663
Abe R, Yamagishi S (2008) AGE-RAGE system and carcinogenesis. Curr Pharm Des 14:940–945
Lorenzi M, Montisano DF, Toledo S, Barrieux A (1986) High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest 77:322–325
Kramer HU, Raum E, Ruter G et al (2012) Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: results from the DIANA study. Cardiovasc Diabetol 11:88
Kirkman MS, Rowan-Martin MT, Levin R et al (2015) Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care 38:604–609
Bertram MY, Vos T (2010) Quantifying the duration of pre-diabetes. Aust N Z J Public Health 34:311–314
Giovannucci E, Harlan DM, Archer MC et al (2010) Diabetes and cancer: a consensus report. Diabetes Care 33:1674–1685
Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P (2013) Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis 16:151–158
Uemura N, Okamoto S, Yamamoto S et al (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345:784–789
Tsukuma H, Hiyama T, Tanaka S et al (1993) Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 328:1797–1801
Szmuilowicz ED, Stuenkel CA, Seely EW (2009) Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol 5:553–558
Shapiro S (2007) Recent epidemiological evidence relevant to the clinical management of the menopause. Climacteric 10(Suppl 2):2–15
Peters SA, Huxley RR, Woodward M (2013) Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke 44:2394–2401
Mongraw-Chaffin ML, Peters SA, Huxley RR, Woodward M (2015) The sex-specific association between BMI and coronary heart disease: a systematic review and meta-analysis of 95 cohorts with 1.2 million participants. Lancet Diabetes Endocrinol 3:437–449
Funding
This study received no external funding. TO is supported by the Japan Society for the Promotion of Science Overseas Research Fellowships. SAEP is supported by a UK Medical Research Council Skills Development Fellowship (MR/P014550/1). MW is a National Health and Medical Research Council of Australia Principal Research Fellow.
Author information
Authors and Affiliations
Contributions
TO searched the scientific literature, did the statistical analyses, participated in data interpretation and drafted the report. SAEP contributed data, did the statistical analyses, participated in data interpretation and made revisions to the draft report. MW conceived the study, contributed data, oversaw the data analyses, participated in data interpretation and made revisions to the draft report. All authors gave final approval of the version to be published and are responsible for the integrity of the work as a whole. TO is the guarantor of this work.
Corresponding authors
Ethics declarations
MW is a consultant to Amgen. Both other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Electronic supplementary material
ESM
(PDF 1.26 MB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ohkuma, T., Peters, S.A.E. & Woodward, M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia 61, 2140–2154 (2018). https://doi.org/10.1007/s00125-018-4664-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4664-5