Abstract
Aim/hypothesis
Combination therapy targeting the major actors involved in the immune-mediated destruction of pancreatic beta cells appears to be an indispensable approach to treat type 1 diabetes effectively. We hypothesised that the combination of an orally active pan-histone deacetylase inhibitor (HDACi: givinostat) with subtherapeutic doses of CD3 antibodies may provide ideal synergy to treat ongoing autoimmunity.
Methods
NOD mice transgenic for the human CD3ε (also known as CD3E) chain (NOD-huCD3ε) were treated for recent-onset diabetes with oral givinostat, subtherapeutic doses of humanised CD3 antibodies (otelixizumab, 50 μg/day, 5 days, i.v.) or a combination of both drugs. Disease remission, metabolic profiles and autoreactive T cell responses were analysed in treated mice.
Results
We demonstrated that givinostat synergised with otelixizumab to induce durable remission of diabetes in 80% of recently diabetic NOD-huCD3ε mice. Remission was obtained in only 47% of mice treated with otelixizumab alone. Oral givinostat monotherapy did not reverse established diabetes but reduced the in situ production of inflammatory cytokines (IL-1β, IL-6, TNF-α). Importantly, the otelixizumab + givinostat combination strongly improved the metabolic status of NOD-huCD3ε mice; the mice recovered the capacity to appropriately produce insulin, control hyperglycaemia and sustain glucose tolerance. Finally, diabetes remission induced by the combination therapy was associated with a significant reduction of insulitis and autoantigen-specific CD8+ T cell responses.
Conclusions/interpretation
HDACi and low-dose CD3 antibodies synergised to abrogate in situ inflammation and thereby improved pancreatic beta cell survival and metabolic function leading to long-lasting diabetes remission. These results support the therapeutic potential of protocols combining these two drugs, both in clinical development, to restore self-tolerance and insulin independence in type 1 diabetes.
Similar content being viewed by others
Introduction
Type 1 diabetes is an autoimmune disease characterised by the destruction of insulin-producing pancreatic beta cells by autoreactive CD4+ and CD8+ T lymphocytes and proinflammatory cytokines. Experimental data indicates that therapeutic efficacy is improved by combining therapies targeting effectors of the autoimmune response and directly modulating beta cell survival rates and function [1,2,3,4,5,6]. Modulation of histone deacetylase (HDAC) activity is considered to be a potential therapeutic strategy for a variety of inflammatory diseases because of its role in energy homeostasis and metabolism, protein folding, transcription and translation. Givinostat (ITF2357) is a novel orally active pan-HDAC inhibitor (HDACi), which has been shown to inhibit the production of inflammatory cytokines by immune cells in vitro and in vivo and reduce inflammatory and autoimmune manifestations [7,8,9]. Interestingly, givinostat also favours beta cell survival and resistance to proinflammatory cytokines (IL-1β, IFNγ, TNF-α) [10,11,12] but so far no clear effect on beta cell regeneration/proliferation has been demonstrated in contrast to other epigenetic modifiers [13,14,15]. In vivo, oral givinostat normalised glycaemia in murine models of streptozotocin-induced diabetes [11] and sustainably reduced diabetes incidence in NOD mice when given at weaning for 100–120 days [12]. Givinostat has not been tested at a later stage of the disease, notably at disease onset, which is characterised by a massive infiltration of pancreatic islets by pathogenic T cells and ongoing beta cell destruction. At this time point, it may still display protective and anti-inflammatory effects on residual pancreatic beta cells and, in combination with T cell targeted immunotherapy, could further promote diabetes remission.
The efficacy of monoclonal CD3-specific antibodies to induce long-lasting disease remission through restoration of immune tolerance has been proven in models of autoimmune diabetes [16]. Using NOD mice, we demonstrated that a short course (5 days) of CD3 antibodies reverted diabetes by resetting efficient immune regulation and a functional balance between pathogenic T cells and regulatory T cells (Tregs) [17,18,19]. Similarly, treatment of NOD mice transgenic for the human CD3ε (also known as CD3E) chain (NOD-huCD3ε) with otelixizumab, a humanised non-Fc-binding CD3 antibody, resulted in sustained disease remission dependent on transferable Treg-mediated tolerance [20]. Phase II and III trials in individuals presenting with new-onset type 1 diabetes have shown that treatment with humanised CD3 antibodies (otelixizumab or teplizumab) efficiently preserved beta cell function, thus decreasing exogenous insulin need [21,22,23,24]. However, the safety profile and long-term efficacy of CD3 antibodies need to be improved. This could be achieved through combination strategies, taking advantage of synergistic effects between individual agents while reducing the dose. These data provide a rationale for testing the efficacy of the combination of anti-human CD3 antibodies and oral givinostat in recent-onset diabetic NOD-huCD3ε mice to induce diabetes remission.
Methods
Mice
NOD-huCD3ε mice were generated in the laboratory (INSERM U1151) and were obtained by speed backcross of BALB/c-huCD3ε mice into the NOD background [20]. NOD-huCD3ε mice develop autoimmune diabetes, as do wild-type NOD mice, generally between 15 and 30 weeks of age. NOD-huCD3ε mice were bred under specific pathogen-free conditions with free access to food and water. Female mice were monitored twice a week for glycosuria (ACCU-CHECK DIABUR test, Roche Diabetes Care, Rotkreuz, Switzerland). Blood glucose levels > 13.9 mmol/l (250 mg/dl) (ACCU-CHECK Performa glucometer, Roche Diabetes Care) confirmed diabetes onset. Experiments were approved by the Ethics Committee of Paris Descartes University (no. 14–076) and the French Ministry of Education and Research (no. 04463.02).
Treatment
Female NOD-huCD3ε mice were treated at diabetes onset by oral givinostat and/or otelixizumab (they were randomly allocated to each group). Givinostat (ITF2357, Selleckchem, Houston, TX, USA) was dissolved in sterile drinking water (5.25 μmol/l of water) containing 1% 2-hydroxypropyl-β-cyclodextrin (Trappsol HBP, CTD Holdings, Alachua, FL, USA), as previously described [12], and was continuously administered. Otelixizumab [25] was administered i.v. at 100 μg/day (full dose) or 50 μg/day (subtherapeutic dose) for five consecutive days. Combined treatment consisted of subtherapeutic doses of otelixizumab (50 μg/day) and oral givinostat.
Flow cytometry
Antimouse CD4 (GK1.5, 1/300), CD8 (53-6.7, 1/300), CD3 (145 2C11, 1/200), CD19 (1D3, 1/400), CD25 (PC61, 1/200) and IL-10 (JES5-16E3, 1/50) and anti-human CD3 antibodies were from BD Biosciences (Le Pont de Claix, France). Antimouse FOXP3 antibodies (FJK-16S, 1/200) were from eBioscience (Life Technologies, Saint-Aubin, France). Detection of autoantigen-specific CD8+ T cells was performed using allophycocyanin (APC)-labelled class I MHC (H-2Kd) tetramers carrying the proinsulin (PI)-B15–23 or the islet-specific glucose-6-phosphatase catalytic subunit related protein (IGRP)206–214 peptides (provided by the NIH Tetramer Core Facility, Atlanta, GA, USA). Briefly, cells were stained with APC-labelled tetramers (0.5 μl/50 μl PBS per well) at 4°C for 1 h. After two washes, cell surface antibodies were added for 20 min of incubation. Cells were analysed on a FACS CANTO II cytometer (BD Biosciences) using the FlowJo software (FlowJo, Ashland, OR, USA).
Pancreatic islet isolation and culture for cytokine production
Pancreatic islets from NOD-huCD3ε mice were separated by density gradient centrifugation (Histopaque, Sigma-Aldrich, Lyon, France) after in situ digestion with collagenase P (Roche Diagnostics, Mannheim, Germany). Then, islets were cultured for 48 h in RMPI medium 10% FCS in 96-well plates (25 islets per well). Supernatant fractions were harvested and IL-6, TNF-α, IL-1β, IL-10 cytokines were detected by ELISA (DuoSet kit, R&D Systems, Abingdon, UK).
IFNγ ELISpot
Polyvinylidene fluoride (PVDF) plates (Merck Millipore, Guyancourt, France) were coated with anti-IFNγ capture antibody (U-CyTech, Utrecht, the Netherlands) as previously described [26]. Splenocytes were cultured at 2.5 × 105 per well. The CD8+ T cell epitopes IGRP206-214 and PI-B15–23 were used at 7 μmol/l. A CD3 antibody (145 2C11, 1 μg/ml) was used as a positive control. After a 20 h culture, IFNγ was detected using a biotinylated anti-IFNγ antibody, streptavidin-horseradish peroxidase and SigmaFAST NBT-BCIP (Sigma-Aldrich). IFNγ spot readouts were expressed as spot-forming units (SFUs)/106 cells.
Histology
Pancreases were fixed in 4% formalin and paraffin embedded, and 4 μm pancreatic sections were stained with haematoxylin/eosin. Six sections for each pancreas were scored for islet infiltration (no infiltration/peri-insulitis/invasive insulitis) by experimenters blind to group assignment.
IPGTT
Mice were fasted for 15 h before intraperitoneal injection with d-glucose (2 g/kg body weight). Blood glucose was measured at baseline and after 15, 30, 60 and 120 min. Glucose clearance was defined as the percentage decrease in blood glucose 30 min after the peak (T30). In separate experiments, serum insulin levels were determined at 0, 15, 30, 60 and 120 min after glucose injection using a mouse insulin ELISA kit (Mercodia, Uppsala, Sweden).
Statistical analysis
All statistical analysis was performed using Graphpad Prism 6 software (Graphpad Software, La Jolla, CA, USA). Data are expressed as mean ± SEM. The occurrence of diabetes/remission was plotted using the Kaplan–Meier method. Statistical comparison of the curves was performed using the logrank (Mantel–Cox) test. The Student’s t test, Mann–Whitney test or two-way ANOVA were used as appropriate. A p value < 0.05 was considered to be statistically significant.
Results
Givinostat preserves residual beta cell function in recent-onset diabetes in NOD-huCD3ε mice through a local anti-inflammatory effect
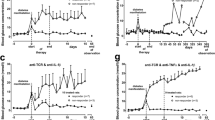
We first evaluated in transgenic NOD-huCD3ε mice the impact of oral givinostat therapy on recent-onset diabetes (diabetic for < 3 days). We observed that givinostat (20–25 μg/day) slowed progression towards severe hyperglycaemia (Fig. 1a), and only 34% of the treated NOD-huCD3ε mice presented blood glucose levels > 33.3 mmol/l 3 weeks after diabetes onset compared with 100% of untreated mice (Fig. 1b). We further analysed treated mice according to their blood glucose level at diabetes diagnosis. The protective effect of givinostat was greater when the treatment was applied in diabetic mice with blood glucose levels of 13.9–22.2 mmol/l (mean 16.9 ± 1.3 mmol/l) (Fig. 1c). In this subgroup, only 20% of mice exhibited hyperglycaemia > 33.3 mmol/l after 3 weeks of follow-up compared with 80% of mice with blood glucose levels > 22.2 mmol/l at the beginning of treatment (Fig. 1d).
Treatment of recent-onset diabetes in NOD-huCD3ε mice with oral givinostat slowed progression towards severe hyperglycaemia and reduced in situ islet inflammatory cytokines. (a) Mean ± SEM blood glucose in diabetic NOD-huCD3ε mice left untreated (squares, n = 9) or continuously treated with oral givinostat (circles, n = 13); ***p < 0.001. (b) Percentage of NOD-huCD3ε mice untreated or treated with oral givinostat and exhibiting blood glucose levels > 33.3 mmol/l; *p < 0.05. (c, d) Diabetic NOD-huCD3ε mice treated with givinostat were divided into subgroups of mice showing either low (13.9–22.2 mmol/l; white circles, n = 6) or high (> 22.2 mmol/l; black triangles, n = 7) blood glucose levels. (c) Mean ± SEM blood glucose in each subgroup; **p < 0.01. (d) Percentage of mice reaching blood glucose levels > 33.3 mmol/l in each subgroup; *p < 0.05. (e–h) Cytokine secretion. Pancreatic islets were isolated from 12-week (wk)-old non-diabetic, untreated diabetic or givinostat (Givi)-treated diabetic NOD-huCD3ε mice (after 3–4 weeks of treatment) (n = 5–9 per group). Islets were cultured at 37°C for 48 h and supernatant fractions were collected. Concentrations of (e) IL-1β, (f) IL-6, (g) TNF-α and (h) IL-10 were determined by ELISA; *p < 0.05
To probe for the local anti-inflammatory properties of givinostat, we quantified the production of IL-1β, TNF-α and IL-6 detected in pancreatic islets recovered from treated NOD-huCD3ε or control mice. In vivo, givinostat monotherapy significantly reduced intra-islet IL-1β, TNF-α and IL-6 levels, compared with untreated diabetic mice, to a level similar to that found in the pancreatic islets from 12-week-old non-diabetic NOD-huCD3ε mice (Fig. 1e–g). In contrast, production of the immunomodulatory cytokine IL-10 was significantly increased after oral givinostat (Fig. 1h). In line with these results, we found that mice treated with givinostat monotherapy showed less invasive insulitis compared with untreated diabetic mice (33% vs 61%, p = 0.002) (Fig. 2b).
Combination of subtherapeutic dose of otelixizumab with givinostat induces remission of diabetes in NOD-huCD3ε mice. (a) Remission of recent-onset diabetes in NOD-huCD3ε mice left untreated (n = 16) or after treatment with full-dose otelixizumab (Otelixi, 5 × 100 μg, n = 23), subtherapeutic otelixizumab (5 × 50 μg, n = 15), oral givinostat (Givi, n = 13) or otelixizumab (5 × 50 μg) + givinostat (n = 26) (p < 0.001, p < 0.001, p = 0.59 and p < 0.001 vs untreated diabetic mice, respectively; p < 0.05 subtherapeutic otelixizumab vs otelixizumab + givinostat). (b) Pancreases were recovered from 12-week (wk)-old non-diabetic (n = 4) and untreated diabetic (Diab; n = 8) NOD-huCD3ε mice and from NOD-huCD3ε mice treated with otelixizumab (50 μg) (n = 15), givinostat (n = 9) or otelixizumab + givinostat (n = 19) (8 weeks after diabetes onset and CD3 antibody treatment). Pancreases were fixed and stained with haematoxylin and eosin. Pancreatic islets were scored for the presence of mononuclear cell infiltration and the percentage of intact islets (white bars) or islets showing peripheral (grey bars) or invasive insulitis (black bars) was calculated
Givinostat synergises with otelixizumab to induce remission of diabetes in NOD-huCD3ε mice
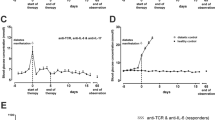
Next we evaluated whether combining oral givinostat with a short course of subtherapeutic doses of anti-human CD3 antibodies (otelixizumab) improved rates of hyperglycaemia reversal in new-onset diabetes in NOD-huCD3ε mice. Full-dose otelixizumab (100 μg/day) induced sustained disease remission in 74% of the treated mice (n = 23) (Fig. 2a). Conversely, continuous administration of givinostat alone (20–25 μg/day) normalised hyperglycaemia in only 8% of mice (1/13). We defined a subtherapeutic dose of otelixizumab (half dose: 50 μg/day) that induced 47% remission (n = 15). Combining this subtherapeutic dose of otelixizumab with givinostat significantly improved rates of hyperglycaemia reversal, which was achieved in 80% of treated NOD-huCD3ε mice (n = 26) (p < 0.0001 between untreated diabetic mice and otelixizumab + givinostat-treated mice) (Fig. 2a). This effect was sustained, lasting at least 8 weeks after therapy. Progressive reversal of hyperglycaemia started between 7 and 15 days after treatment initiation and the mean blood glucose level of mice in remission was reduced from 18.7 ± 3.2 mmol/l prior to treatment to 8.6 ± 1.8 mmol/l. No signs of toxicity related to treatment with otelixizumab + givinostat (weight loss, altered behaviour, fur loss, or urine and stool discoloration) were observed. Histological analysis revealed that NOD-huCD3ε mice treated with otelixizumab + givinostat had significantly fewer islets showing destructive insulitis compared with diabetic mice (19% vs 61%, p < 0.001) (Fig. 2b). They also displayed reduced peripheral insulitis compared with NOD-huCD3ε mice treated with otelixizumab monotherapy (18% vs 32%, p = 0.039).
To investigate whether continuous administration of givinostat was required to sustain the therapeutic effect, oral givinostat was withdrawn after 3 or 9 weeks of treatment. In both situations, remission of diabetes was maintained in NOD-huCD3ε mice for at least 6 additional weeks (see ESM Fig. 1a,b).
Otelixizumab + givinostat treatment rescues islet beta cell function and recovers glucose tolerance and insulin secretion
To assess the metabolic impact of the different treatments on beta cell function, we performed IPGTTs. As expected, glucose tolerance and insulin secretion were completely abnormal in untreated diabetic NOD-huCD3ε mice (glycaemia > 13.9 mmol/l at all time points analysed) (Fig. 3a–c). Mice that achieved remission with subtherapeutic doses of otelixizumab regained responsiveness to a glucose challenge and showed better blood glucose clearance compared with mice treated with subtherapeutic doses of otelixizumab that remained diabetic (hyperglycaemic). Importantly, we observed that NOD-huCD3ε mice in remission for 8–10 weeks after otelixizumab + givinostat therapy improved their metabolic profile, insulin secretion (fasting and after glucose challenge) and glucose clearance (Fig. 3a–c). The combination therapy normalised fasting glycaemia (mean 4.7 ± 0.9 mmol/l) and T120 blood glucose (mean 7.8 ± 1.3 mmol/l) (Fig. 3a) and restored efficient insulin secretion compared with diabetic mice (p = 0.032 at 15 min) (Fig. 3c). Blood glucose clearance reached levels similar to that of 12–16-week-old non-diabetic mice and of mice showing impaired glucose tolerance (35% vs 44% and 33%, respectively) (Fig. 3b). Impaired glucose tolerance was defined by a 1 h blood glucose level > 10 mmol/l and normal fasting blood glucose in NOD-huCD3ε mice that did not present glycosuria. The combination treatment also restored insulin secretion better than otelixizumab monotherapy in mice in remission (non-significant trend) (Fig. 3c). Although givinostat-treated mice showed very low insulinaemia and altered glucose clearance, they had normal fasting blood glucose (mean 5.2 ± 1.3 mmol/l) and better controlled hyperglycaemia at T120 (mean blood glucose 14.8 ± 2.6 mmol/l) compared with untreated diabetic mice (mean 21.9 ± 1.9 mmol/l) (Fig. 3a).
Otelixizumab + givinostat combination therapy improves glucose tolerance and insulin secretion in NOD-huCD3ε mice. (a) IPGTTs were performed with glucose 2 g/kg in 12–16-week (wk)-old non-diabetic NOD-huCD3ε mice (n = 18) or diabetic NOD-huCD3ε mice left untreated (diabetic, n = 16) or treated with givinostat (Givi, n = 11), otelixizumab (Otelixi, 50 μg) (n = 4 in remission; n = 3 still diabetic) or otelixizumab + givinostat (n = 8 in remission). Transgenic mice showing impaired glucose tolerance were also included (n = 4). Data are presented as mean ± SEM measured 15, 30, 60 and 120 min after glucose injection; *p < 0.05 and **p < 0.01 for otelixizumab + givinostat and otelixizumab (50 μg) groups vs untreated group, respectively, at T120; *p < 0.05 for givinostat-treated mice vs untreated diabetic mice at T120. (b) Measurement of glucose clearance (i.e. percentage decrease in blood glucose level 30 min after the peak). Data are presented as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001. (c) Insulin secretion was measured in the serum of treated or untreated NOD-huCD3ε mice subjected to an IPGTT; *p < 0.05 and **p < 0.01 vs untreated diabetic mice at T15
The otelixizumab + givinostat combination reduces T cell response to beta cell autoantigens
CD8+ T cell IFNγ responses (ELISpot) towards the immunodominant pancreatic beta cell peptides IGRP206-214 and PI15-23 were significantly reduced in NOD-huCD3ε mice treated with otelixizumab + givinostat compared with untreated diabetic or givinostat-treated mice (Fig. 4a,b). Of note, otelixizumab monotherapy decreased responses to IGRP206-214 but not PI15-23. Responses to polyclonal stimulation (CD3 antibodies 145 2C11) were comparable between all groups (Fig. 4c). Pancreatic lymph nodes (pLNs) of NOD-huCD3ε mice treated with the combination therapy also displayed decreased proportions and absolute numbers of autoreactive CD8+ T cells specific for the PI15-23 or IGRP206-214 peptides compared with the other groups (tetramer staining, Fig. 4d–g). Variable but significant numbers of PI15-23-specific CD8+ T cells were detected in mice treated with otelixizumab alone (statistically comparable with that measured in untreated mice).
The combination therapy reduces autoantigen-specific CD8+ T cell responses. (a–c) Specific IFNγ responses (ELISpot) of spleen cells from NOD-huCD3ε mice untreated or treated with givinostat (Givi) and/or otelixizumab (Otelixi) towards the CD8+ T cell restricted epitopes (a) PI15-23 or (b) IGRP206-214. (c) A CD3 antibody was used as a positive control. Treated mice were killed 4–8 weeks after diabetes onset. Data are expressed as SFU/1 × 106 cells (n = 6–13 per group); *p < 0.05, **p < 0.01, ***p < 0.001. (d–g) Percentage and absolute number of CD8+ T cells specific for the autoantigenic peptides (d, e) PI15–23 or (f, g) IGRP206–214 evaluated by MHC class I tetramer (Tet) staining in the pLNs of NOD-huCD3ε mice untreated or treated with otelixizumab and/or givinostat (n = 6–13 per group) (*p < 0.05, **p < 0.01)
Finally, increased proportions (within the CD4+ T cell compartment) of CD4+FOXP3+ T cells (Tregs) were similarly observed in the spleen and pLNs of NOD-huCD3ε mice 1 month after treatment with either otelixizumab + givinostat or otelixizumab alone (ESM Fig. 2a,c). Such an increase was not noticed after givinostat monotherapy. However, absolute numbers of Tregs were not statistically different in all groups tested (ESM Fig. 2b,d). This result is in accordance with the well-known property of CD3 antibodies to preferentially deplete activated effector T cells while sparing Tregs [18, 27, 28]. Two months after otelixizumab treatment, Tregs were returned to normal levels, both in terms of absolute numbers and proportions in the spleen and pLNs of treated mice (ESM Fig. 2a–d).
In connection with the IL-10 production observed in givinostat-treated mice (Fig. 1h), we searched for the presence of regulatory type 1 (Tr1) cells, characterised by co-expression of CD49b and lymphocyte-activation gene 3 (LAG-3) [29] and by their capacity to produce high amounts of IL-10. Very few Tr1 cells were detected in the spleen, pLNs and pancreas of NOD-huCD3ε mice treated with combination therapy or givinostat or otelixizumab monotherapy, or untreated mice (ESM Fig. 3a). In addition, total IL-10 production by CD4+ T cells was very low and did not increase following administration of givinostat and/or otelixizumab (ESM Fig. 3b).
Discussion
Data gathered from experimental and clinical immune intervention studies point to the necessity of combination therapy to successfully cure type 1 diabetes. This could be achieved by simultaneously targeting different key immune players, such as antigen-presenting cells and T cells (pathogenic and/or Tregs), and by administering drugs that improve beta cell function/mass together with immune modulators. Such a strategy has been shown to be successful as the combination of exendin-4, a glucagon-like peptide receptor agonist, with CD3 monoclonal antibodies or anti-lymphocytes serum improved diabetes remission rates in NOD mice by enhancing recovery of residual beta cells as well as insulin content and release from beta cells [1, 2].
In the present study, we took advantage of the well-described protective effect of givinostat, a HDACi, on pancreatic beta cells. Indeed, in mouse, rat and human islets and in beta cell lines, givinostat prevents cytokine-induced beta cell death and preserves beta cell function by reducing the expression of the IFNγ-inducible chemokines CXCL9 and CXCL10, expression of nitric oxide synthase (iNOS) and production of nitric oxide through the inhibition of NF-κB transcriptional activity and extracellular signal-regulated kinase (ERK) phosphorylation [11, 12, 30,31,32]. Accordingly, we observed that givinostat could, by itself, improve glucose tolerance in recent-onset diabetic NOD-huCD3ε mice, which is probably related to the combined anti-inflammatory effects exerted at both the immune cell and islet levels [12] and/or to an increased sensitivity to insulin. Indeed, givinostat, like most agents that inhibit HDAC, has demonstrated potent anti-inflammatory properties both in vitro and in vivo. Givinostat inhibited the production of proinflammatory cytokines by concanavalin A-stimulated splenocytes or by lipopolysaccharide (LPS)-stimulated peritoneal macrophages and reduced LPS-induced in vivo systemic inflammation [9, 11, 12]. Similarly, we found that ex vivo intraislet secretion of IL-1β, IL-6 and TNF-α by immune cells was decreased in NOD-huCD3ε mice treated with oral givinostat; in contrast to IL-10, which was increased. Interestingly, Il10 gene expression was increased in the spleen of NOD mice treated with another pan-HDACi (vorinostat) from weaning [12]. The capacity of HDAC inhibitors to promote IL-10 while inhibiting inflammatory cytokines was recently confirmed in vitro on epithelial, fibroblast and myogenic cell lines, as well as in vivo in response to silicone breast implants in mice [33]. Although the role of IL-10 in diabetes remission remains to be determined, our results suggest that pancreatic islets switched from a proinflammatory to an anti-inflammatory microenvironment upon givinostat treatment.
Our previous work suggests that givinostat does not exert its actions at the histone level as we did not detect any effects of HDACi treatment on histone acetylation in NOD mice [12]. In addition, we found that givinostat reduced beta cell and peritoneal macrophage inflammatory gene expression, contrary to the dogmatic view that histone hyperacetylation leads to an open chromatin structure and transcriptional activation [12]. Transcriptomic analysis of HDACi-treated beta cells and macrophages strongly pinpoints NF-κB as the key regulating node and we found that the NF-κB subunit p65 was hyperacetylated in beta cells exposed to givinostat [12]. This hyperacetylation prevented p65 from binding inflammatory gene promoters providing a mechanistic explanation for the above observations. The anti-inflammatory effects of HDACi have been demonstrated in vitro in model systems using either insulin-producing cells, isolated islets devoid of immune cells or purified leukocytes, strongly indicating that the HDACi anti-diabetic action is exerted at both the immune system and islet level [10,11,12, 30, 31, 34].
Although oral givinostat efficiently targeted islet inflammation and promoted beta cell function, it was not sufficient to reverse established disease in NOD-huCD3ε mice. Sustained diabetes remission was achieved when givinostat was combined with a short course of subtherapeutic doses of humanised CD3 antibodies (otelixizumab). Such a combination regimen favoured pancreatic beta cell survival, secretory function and resistance to inflammation. It improved the overall metabolic status of treated mice that recovered their capacity to secrete increased amounts of insulin, control hyperglycaemia and sustain glucose tolerance.
In addition, our data suggest that givinostat treatment improved responses to CD3 antibody immune therapy. Administration of subtherapeutic doses of otelixizumab combined with givinostat drastically reduced CD8+ T cell IFNγ responses towards the immunogenic PI15-23 and IGRP206-214 peptides, and very low frequencies and absolute numbers of CD8+ T cells specific for these peptides were detected in the pLNs compared with untreated diabetic NOD-huCD3ε mice. Interestingly, as already described [20], responsiveness towards PI (but not IGRP) is sustained after CD3 antibody monotherapy; NOD-huCD3ε mice treated with otelixizumab alone still display significant numbers of PI-specific CD8+ T cells and potent IFNγ responses specific to PI. Thus, the combination with givinostat contributed to reduce tissue inflammation and provided a more complete and sustained downregulation of autoreactive T cell responses. Such an impact on autoimmune responses was not reported when CD3 antibodies were combined with exendin-4 [2]. Consistent with recent publications from our group and others, this unresponsiveness may be associated with the presence of anergic or exhausted-like T cells expressing inhibitory receptors, such as programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1)/LAG-3/T cell immunoreceptor with Ig and ITIM domains (TIGIT), as well as the transcription factor eomesodermin (Eomes) [35,36,37]. Another field of investigation concerns the impact of the combination therapy on glucose metabolism as CD3 antibodies have been shown to downregulate the expression of components of the glycolysis pathway, such as the glucose transporter GLUT1, in effector T cells and HDACi are able to inhibit GLUT1-mediated glucose transport [37, 38].
In our model, we did not notice any significant effect of givinostat on CD4+FOXP3+ Tregs. Absolute numbers and proportions are similar in untreated and givinostat-treated NOD-huCD3ε mice. We and others have previously demonstrated that FOXP3+ Tregs are preserved from the CD3 antibody depleting effect, which mostly targets activated effector CD4+ and CD8+ T cells [18, 27, 28, 37]. Consequently, Treg frequency in the CD4+ T cell compartment increases after CD3 antibody therapy. This is what we observed in the present study 1 month after treatment with otelixizumab alone or in combination; however, absolute Treg numbers were constant. By 2 months (i.e. a time point where the T cell compartment has fully reconstituted), Treg proportions and absolute numbers were similar in all groups, showing that givinostat did not further promote Treg expansion in contrast to what was reported when it was administered at weaning [12]. Such a difference may be related to the therapeutic window. We also did not find any evidence in the spleen or pLNs for the presence of Tr1 cells, another regulatory CD4+ T cell subset induced in response to IL-10 and that act in an IL-10-dependent manner [29, 39, 40]. The possible and preferential induction of Tr1 cells in the small intestine of treated mice remains to be investigated.
Therefore, our data indicate that givinostat and otelixizumab acted in synergy to restore immune tolerance within pancreatic islets where, complementary to otelixizumab-mediated elimination of pathogenic effector T cells, givinostat favoured beta cell survival and recovery of secretory function in a non-inflammatory environment. A continuous supply of givinostat was not required to sustain the therapeutic effect, suggesting a critical role in the early phase of tolerance induction at the time of CD3 antibody therapy.
In terms of clinical translation, the combination of an HDACi and CD3 antibodies offers several advantages. First, humanised CD3 antibodies have been tested in individuals with type 1 diabetes. Promising results have been obtained in phase II and III trials in terms of preservation of the insulin secretory capacity, resulting in reduced insulin need [21,22,23,24]. Second, givinostat is administered orally and its therapeutic effect is currently being evaluated in a wide range of indications. A phase II study in active systemic onset juvenile idiopathic arthritis showed that a low oral dose of givinostat achieved significant reductions in joint and systemic inflammation with no organ toxicity [41]. In addition, there is a growing interest in the use of an isoform-selective HDACi, which may have a more tailored and safer effect than a pan-HDACi. HDAC3 inhibition, using siRNA knockdown or pharmacological agents, was shown to be effective for protecting pancreatic beta cells from cytokine-induced apoptosis and improved both islet size and insulin sensitivity and secretion without haematological adverse effects [34, 42,43,44,45]. Accordingly, conditional HDAC3 ablation in beta cells of adult mice increased insulin secretion and improved glucose metabolism, supporting the finding that HDAC3 is a major regulator of gene transcription in beta cells [46]. Furthermore, HDAC7 may also be a potential target as a recent study showed that HDAC7 was overexpressed in the islets of individuals with type 2 diabetes, and this correlated with impaired insulin content and secretion [47]. HDAC7 inhibition restored glucose-stimulated insulin production in an HDAC7-overexpressing beta cell line [47].
In conclusion, our data strengthen the therapeutic potential of a small molecule HDACi in resolving chronic autoimmune inflammation in type 1 diabetes and further consolidating the therapeutic efficacy of combination treatments that simultaneously target T cells and protect pancreatic beta cells.
Abbreviations
- APC:
-
Allophycocyanin
- HDAC:
-
Histone deacetylase
- FOXP3:
-
Forkhead box P3
- HDACi:
-
Histone deacetylase inhibitor
- IGRP:
-
Islet-specific glucose-6-phosphatase catalytic subunit related protein
- LAG-3:
-
Lymphocyte-activation gene 3
- PI:
-
Proinsulin
- pLN:
-
Pancreatic lymph node
- SFU:
-
Spot-forming unit
- Tr1:
-
Regulatory type 1 cell
- Treg:
-
Regulatory T cell
References
Ogawa N, List JF, Habener JF, Maki T (2004) Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes 53:1700–1705
Sherry NA, Chen W, Kushner JA et al (2007) Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 148:5136–5144
Xue S, Posgai A, Wasserfall C et al (2015) Combination therapy reverses hyperglycemia in NOD mice with established type 1 diabetes. Diabetes 64:3873–3884
Ding L, Gysemans CA, Stange G et al (2014) Combining MK626, a novel DPP-4 inhibitor, and low-dose monoclonal CD3 antibody for stable remission of new-onset diabetes in mice. PLoS One 9:e107935
Tian J, Dang H, Nguyen AV, Chen Z, Kaufman DL (2014) Combined therapy with GABA and proinsulin/alum acts synergistically to restore long-term normoglycemia by modulating T cell autoimmunity and promoting beta-cell replication in newly diabetic NOD mice. Diabetes 63:3128–3134
Ablamunits V, Henegariu O, Hansen JB et al (2012) Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes 61:145–154
Glauben R, Batra A, Fedke I et al (2006) Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol 176:5015–5022
Joosten LA, Leoni F, Meghji S, Mascagni P (2011) Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med 17:391–396
Leoni F, Fossati G, Lewis EC et al (2005) The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med 11:1–15
Larsen L, Tonnesen M, Ronn SG et al (2007) Inhibition of histone deacetylases prevents cytokine-induced toxicity in beta cells. Diabetologia 50:779–789
Lewis EC, Blaabjerg L, Storling J et al (2011) The oral histone deacetylase inhibitor ITF2357 reduces cytokines and protects islet beta cells in vivo and in vitro. Mol Med 17:369–377
Christensen DP, Gysemans C, Lundh M et al (2014) Lysine deacetylase inhibition prevents diabetes by chromatin-independent immunoregulation and beta-cell protection. Proc Natl Acad Sci U S A 111:1055–1059
Xu G, Stoffers DA, Habener JF, Bonner-Weir S (1999) Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48:2270–2276
Stoffers DA, Kieffer TJ, Hussain MA et al (2000) Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748
Fu W, Farache J, Clardy SM et al (2014) Epigenetic modulation of type-1 diabetes via a dual effect on pancreatic macrophages and beta cells. eLife 3:e04631
Chatenoud L, Bluestone JA (2007) CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol 7:622–632
Chatenoud L, Thervet E, Primo J, Bach JF (1994) Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 91:123–127
Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L (2003) TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9:1202–1208
You S, Candon S, Kuhn C, Bach JF, Chatenoud L (2008) CD3 antibodies as unique tools to restore self-tolerance in established autoimmunity their mode of action and clinical application in type 1 diabetes. Adv Immunol 100:13–37
Kuhn C, You S, Valette F et al (2011) Human CD3 transgenic mice: preclinical testing of antibodies promoting immune tolerance. Sci Transl Med 3:68ra10
Keymeulen B, Vandemeulebroucke E, Ziegler AG et al (2005) Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352:2598–2608
Keymeulen B, Walter M, Mathieu C et al (2010) Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 53:614–623
Herold KC, Hagopian W, Auger JA et al (2002) Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346:1692–1698
Sherry N, Hagopian W, Ludvigsson J et al (2011) Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 6736:60931–60938
Friend PJ, Hale G, Chatenoud L et al (1999) Phase I study of an engineered aglycosylated humanized CD3 antibody in renal transplant rejection. Transplantation 68:1632–1637
Enee E, Martinuzzi E, Blancou P, Bach JM, Mallone R, van Endert P (2008) Equivalent specificity of peripheral blood and islet-infiltrating CD8+ T lymphocytes in spontaneously diabetic HLA-A2 transgenic NOD mice. J Immunol 180:5430–5438
Penaranda C, Tang Q, Bluestone JA (2011) Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol 187:2015–2022
Besancon A, Baas M, Goncalves T et al (2017) The Induction and maintenance of transplant tolerance engages both regulatory and anergic CD4+ T cells. Front Immunol 8:218
Gagliani N, Magnani CF, Huber S et al (2013) Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 19:739–746
Dahllof MS, Christensen DP, Lundh M et al (2012) The lysine deacetylase inhibitor Givinostat inhibits beta-cell IL-1beta induced IL-1beta transcription and processing. Islets 4:417–422
Dahllof MS, Christensen DP, Harving M, Wagner BK, Mandrup-Poulsen T, Lundh M (2015) HDAC inhibitor-mediated beta-cell protection against cytokine-induced toxicity is STAT1 Tyr701 phosphorylation independent. J Interf Cytokine Res 35:63–70
Leus NG, Zwinderman MR, Dekker FJ (2016) Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-kappaB-mediated inflammation. Curr Opin Chem Biol 33:160–168
Di Liddo R, Valente S, Taurone S et al (2016) Histone deacetylase inhibitors restore IL-10 expression in lipopolysaccharide-induced cell inflammation and reduce IL-1beta and IL-6 production in breast silicone implant in C57BL/6J wild-type murine model. Autoimmunity. https://doi.org/10.3109/08916934.2015.1134510
Lundh M, Christensen DP, Damgaard Nielsen M et al (2012) Histone deacetylases 1 and 3 but not 2 mediate cytokine-induced beta cell apoptosis in INS-1 cells and dispersed primary islets from rats and are differentially regulated in the islets of type 1 diabetic children. Diabetologia 55:2421–2431
Baas M, Besancon A, Goncalves T et al (2016) TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8+ T cell anergy in transplant tolerance. eLife 5:e08133
Long AA, Thorpe J, DeBerg HA et al (2016) Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol 1:eaai7793
Wallberg M, Recino A, Phillips J et al (2017) Anti-CD3 treatment up-regulates programmed cell death protein-1 expression on activated effector T cells and severely impairs their inflammatory capacity. Immunology 151:248–260
Wardell SE, Ilkayeva OR, Wieman HL et al (2009) Glucose metabolism as a target of histone deacetylase inhibitors. Mol Endocrinol 23:388–401
Groux H, O’garra A, Bigler M et al (1997) A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature 389:737–742
Roncarolo MG, Gregori S, Bacchetta R, Battaglia M (2014) Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol 380:39–68
Vojinovic J, Damjanov N, D’Urzo C et al (2011) Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 63:1452–1458
Chou DH, Holson EB, Wagner FF et al (2012) Inhibition of histone deacetylase 3 protects beta cells from cytokine-induced apoptosis. Chem Biol 19:669–673
Plaisance V, Rolland L, Gmyr V et al (2014) The class I histone deacetylase inhibitor MS-275 prevents pancreatic beta cell death induced by palmitate. J Diabetes Res 2014:195739
Lundh M, Galbo T, Poulsen SS, Mandrup-Poulsen T (2015) Histone deacetylase 3 inhibition improves glycaemia and insulin secretion in obese diabetic rats. Diabetes Obes Metab 17:703–707
Wagner FF, Lundh M, Kaya T et al (2016) An isochemogenic set of inhibitors to define the therapeutic potential of histone deacetylases in beta-cell protection. ACS Chem Biol 11:363–374
Remsberg JR, Ediger BN, Ho WY et al (2017) Deletion of histone deacetylase 3 in adult beta cells improves glucose tolerance via increased insulin secretion. Mol Metab 6:30–37
Daneshpajooh M, Bacos K, Bysani M et al (2017) HDAC7 is overexpressed in human diabetic islets and impairs insulin secretion in rat islets and clonal beta cells. Diabetologia 60:116–125
Acknowledgements
The authors thank M. Bellanger (INSERM U1151, Paris, France) for taking care of the NOD-huCD3ε mouse colony and for providing technical assistance for the experimental mouse work. We are also grateful to E. Panafieu, S. Fonlebeck and Y. Loudin (INSERM U1151, Department of Immunology, Paris, France) for mouse production and maintenance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
The data generated during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by grants from the JDRF (#1-2011-654), institutional funding from INSERM and University Paris Descartes and also with the support of Fondation Day Solvay and Fondation Centaure. AB was supported by a doctoral fellowship from INSERM and by a grant from the Société Française d’Endocrinologie et Diabétologie Pédiatrique (grant from Novo Nordisk). The funders had no role in study design, data collection, interpretation or decision to submit the work for publication.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
AB designed experiments, acquired and analysed data, and wrote the manuscript. TG and FV designed and performed experiments, and analysed data. MSD provided research material and contributed to the design of the experiments. LC provided critical advice and help in writing the manuscript. TM-P initiated the study with LC and contributed to planning the protocol and reviewed the manuscript. All authors revised the manuscript and approved the final version to be published. SY designed and directed the study, analysed the data and wrote the manuscript. SY is the guarantor of this work.
Electronic supplementary material
ESM Figures
(PDF 290 kb)
Rights and permissions
About this article
Cite this article
Besançon, A., Goncalves, T., Valette, F. et al. Oral histone deacetylase inhibitor synergises with T cell targeted immunotherapy to preserve beta cell metabolic function and induce stable remission of new-onset autoimmune diabetes in NOD mice. Diabetologia 61, 389–398 (2018). https://doi.org/10.1007/s00125-017-4459-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-017-4459-0