Abstract
Aims/hypothesis
Chronic hyperglycaemia, as measured by HbA1c levels, is a major risk factor for atherosclerosis and cardiovascular disease (CVD) in type 1 diabetes. Our aim was to describe the degree to which the effect of HbA1c on the risk of CVD is mediated by its effect on traditional risk factors over time, and how these mediation pathways change over time.
Methods
The DCCT and its observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC), followed 1441 participants for a mean of 27 years, with periodic measurement of HbA1c and risk factors over time. We assessed the proportion of the HbA1c effect on risk of CVD that was mediated through its effects on systolic BP (SBP), pulse rate, triacylglycerols and LDL-cholesterol (LDLc) levels, and how the proportion mediated changed over time.
Results
The association of HbA1c with CVD outcomes was stable over time, while that of traditional risk factors (SBP, pulse rate, triacylglycerols and LDLc) increased. At 10 years of follow-up, the effect of HbA1c on 10 year CVD risk was minimally mediated by SBP (2.7%), increasing to 26% at 20 years. Likewise, from 10 year follow-up to 20 year follow-up, the proportion of HbA1c effect mediated through pulse rate increased from 6.3% to 29.3%, through triacylglycerols from 2.2% to 22.4%, and through LDLc from 9.2% to 30.7%.
Conclusions/interpretation
As participants age, the predictive association of mean HbA1c on subsequent CVD events is increasingly mediated by its effect on standard risk factors. Thus, management of traditional non-glycaemic CVD risk factors may have increasing benefits in an ageing type 1 diabetes population with longstanding hyperglycaemia.
Trial registration: ClinicalTrials.gov NCT00360893 and NCT00360815.
Similar content being viewed by others
Introduction
Despite progress in treatment, the risk of cardiovascular disease (CVD) in type 1 diabetes remains higher compared with that in the age-matched population without diabetes [1, 2]. Over a mean follow-up of 17 years, the DCCT and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, demonstrated that an average of 6.5 years of intensive diabetes therapy, resulting in a mean HbA1c of ~7% (53 mmol/mol) in participants, reduced the risk of any CVD event by 42%, and the risk of non-fatal myocardial infarction or stroke or death from CVD (major atherosclerotic cardiovascular events [MACE]) by 57% compared with conventional therapy, which resulted in a mean HbA1c of ~9% (75 mmol/mol) [3]. The robustness of the protective effect of 6.5 years of intensive therapy was further confirmed by an additional 10 years of follow-up [4]. Over the entire 28 years of follow-up, intensive therapy reduced the incidence of any CVD event by 30% and of MACE by 32% compared with conventional therapy [4], despite similar glycaemic levels for the majority of the EDIC follow-up period.
In order to provide adequate power to detect effects reliably using Cox proportional hazard models, further investigation of risk factors for CVD was embargoed until 100 participants in the former DCCT conventional group had experienced a CVD event. This landmark was reached in 2013 and a formal risk factor analysis was carried out. In a multivariable Cox model that also adjusted for age, duration of diabetes, use of angiotensin-converting-enzyme (ACE) inhibitors and a family history of myocardial infarction, the time-weighted updated DCCT/EDIC mean HbA1c was a stronger predictor of future CVD events than the updated mean systolic BP (SBP), pulse rate, LDL-cholesterol (LDLc), and current triacylglycerols (triacyclglycerol value at time of testing), which also predicted events [5].
These analyses, however, did not allow for changing covariate effects over time, nor did they address whether any of the effect of HbA1c on CVD risk was mediated by the effect of HbA1c on other CVD risk factors and whether this mediated effect varied over time. In this context, mediation analyses are important as poor glycaemic control is associated with dyslipidaemia and higher pulse rate and BP [1, 6], while more intensive glycaemic control is associated with the reversal of these effects [7]. Therefore, we have assessed the temporal changes in the effects of HbA1c and traditional CVD risk factors on the risk of CVD, and investigated potential pathways by which changes in CVD risk factors over time could mediate the effects of HbA1c on the risk of CVD during the 28 year follow-up of the DCCT/EDIC cohort.
Methods
The methods of the DCCT and EDIC study have been described in detail [8]. Briefly, a total of 1441 participants with type 1 diabetes were randomly assigned to receive either intensive therapy (n = 711) with the goal of lowering blood glucose levels as close to the ‘non-diabetic’ range as safely as possible, or to conventional therapy (n = 730) aimed at avoiding symptoms related to hyper- or hypoglycaemia without specific glucose targets. The DCCT ended in 1993, after an average of 6.5 years of follow-up, and all participants were offered intensive therapy and referred to their healthcare providers for subsequent diabetes care. In 1994, 98% of the surviving DCCT cohort enrolled in the EDIC follow-up observational study and, after an additional 20 years of follow-up, 94% of the cohort survivors are still actively participating. The DCCT and EDIC protocols were approved by the institutional review boards of all participating centres and all participants provided written informed consent.
Cardiovascular risk factors
The analyses presented are based on data obtained at annual visits during both the DCCT and EDIC study, which included a detailed medical history, physical examination (e.g. BP and pulse rate) and collection of bio-specimens (e.g. blood samples). Recognised and putative CVD risk factors were evaluated by standardised methods [8, 9]. HbA1c was measured using high-performance liquid chromatography, quarterly during the DCCT and annually during EDIC. Fasting lipids (triacylglycerols, total cholesterol and HDL-cholesterol) were measured in the central laboratory annually during DCCT and every other year during EDIC, and LDLc was calculated using the Friedewald equation [10]. The updated weighted mean of a covariate (e.g. HbA1c) measured at different intervals in DCCT vs EDIC was computed using all values up to a particular visit, with weights proportional to the time intervals between measurements.
Cardiovascular outcomes
CVD events were ascertained based on the medical history and electrocardiogram and relevant available medical records, and were adjudicated by a committee masked to DCCT treatment group and HbA1c levels. The composite CVD outcome was defined as time to the first occurrence of CVD death, non-fatal myocardial infarction, non-fatal stroke, subclinical myocardial infarction on ECG, angina confirmed by ischaemic changes with exercise tolerance testing or by clinically significant obstruction on coronary angiography, revascularisation (with angioplasty or coronary artery bypass) or congestive heart failure (paroxysmal nocturnal dyspnea, orthopnea or marked limitation of physical activity caused by heart disease) [11]. All participants with an initial CVD event that occurred prior to 31 December 2013 were included in these analyses.
Statistical analysis
The availability of longitudinal measurements throughout the DCCT/EDIC study allows for the dynamic evaluation of the temporal changes in the covariate effects on the risk of CVD events over time. Aalen additive hazard models [12] assessed the effects of covariates at 10 years of follow-up on the risk of CVD during the subsequent 10 years of follow-up (years 10 to 20). Only CVD events within this 10 year follow-up period were included. Participants with CVD events that had occurred prior to the 10 year visit were excluded and participants who did not experience an event within the subsequent 10 years were administratively right censored after year 20. Additional models then assessed the effects of covariates measured up to the 11 year visit on the risk of CVD during the subsequent 10 year interval (years 11–21). Likewise, models assessed effects of covariates measured up to year 12 on CVD outcomes during years 12–22, then the effects of those measured up to year 13 during years 13–23, up to models that assessed the effects of covariates measured at year 20 on the risk of CVD during years 20–30. For each participant, the 10 year period of risk was computed to the day from the beginning of the period, e.g. for the 11–21-year models, the period of risk for a given participant spanned the time from 11.00 years after the participant’s date of randomisation to 21.00 years after. The models employed the covariate values up to the most recent visit prior to the start of the 10 year period.
Based on our recent CVD risk factor assessment [5] that identified the most significant risk factors from an extensive panel of previously established and putative risk factors (‘full model’, ESM Table 1), models employed the updated mean values of HbA1c, SBP, pulse rate, LDLc and the ‘current’ value of log e (triacylglycerols) at the beginning of each 10 year interval as fixed covariates. For example, the 10–20 year models employed the updated mean or current covariate value at year 10, the 11–21 year models used covariate values at year 11, and so on. Similar to the Framingham [13] and American College of Cardiology (ACC)/American Heart Association (AHA) [14] scores, all analyses herein address covariate effects on the risk of CVD within a 10 year time horizon. All analyses adjusted for age and duration of diabetes, both being well-known risk factors for CVD.
The AUC of the receiver operating characteristic (ROC) curve describes the predictive ability of a variable or a model, allowing for censored data [15]. It represents the probability that a participant who experienced an event has a higher risk score than a participant who is event free; AUC = 0.5 represents a risk factor that is not associated with the outcome, while AUC = 1 corresponds to perfect prediction.
Models with covariate (SBP, LDLc, triacylglycerols or pulse rate), age and diabetes duration (‘Model 1’), age and diabetes duration only (‘Model 2’), HbA1c, age and diabetes duration (‘Model 3’) and the full model (‘Model 4’, ESM Table 1) were considered. For each model, the AUC was computed for each of the 10 year models (10–20 years, 11–21 years, etc.) to describe the changes over time in the predictive effect of that covariate on the subsequent 10 year risk of CVD. More specifically, using the 10–20 year interval as an example, the model with covariates measured at year 10 was used to compute the AUC for CVD events in year 10 alone, then for events in years 10–11, then years 10–12, etc.; the AUC value at a particular time point (say, year 15) describes the ability of the model with covariates evaluated at year 10 to predict CVD events up to that time point (i.e. year 15). The AUC values are presented for descriptive purposes only and no formal statistical tests were performed to compare the AUC values across different models.
Mediation analyses [16] were then employed to investigate whether the predictive effect of HbA1c on CVD risk in each 10 year model could be explained by another covariate. Using SBP as an example, the total effect of mean HbA1c on CVD was decomposed as the sum of the direct effect of HbA1c (effect of HbA1c on CVD) and the indirect effect of HbA1c (effect of HbA1c on CVD mediated through its effects on SBP) (Fig. 1). The mediation proportion (also called proportion explained) is the ratio of the indirect effect to the total effect (proportion explained = indirect effect/total effect). Similar to the AUC calculations, the mediation pathways were assessed in a dynamic fashion in successive 10 year models (10–20, 11–21, etc.).
The single mediator model, in which HbA1c has a direct effect on CVD outcomes through the HbA1c to CVD pathway, and an indirect effect on CVD is mediated by the effect of HbA1c on SBP through the HbA1c to SBP to CVD pathway. The total HbA1c effect on CVD is the sum of the direct and indirect effects. The parameters α1 and γ1/γ2 describe the effect of HbA1c on SBP and the effects of HbA1c and SBP on the risk of CVD, respectively (see ESM Mediation Analysis for details)
Linear regression models were employed to assess the effect of mean HbA1c on potential mediators (such as SBP), while Aalen additive hazard models were used for the time-to-event CVD outcome [12]. The direct and indirect effects are functions of the coefficients in the two models (see ESM Mediation Analysis). In this case, the Aalen model was chosen for use rather than the Cox proportional hazards model because the resulting effects (described above) have a direct interpretation in terms of the number of CVD events attributable to the corresponding pathways.
Results
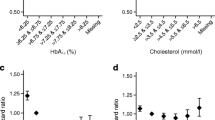
The number of participants at risk of CVD events in the DCCT/ EDIC cohort are presented for each 10 year interval in ESM Table 2. There were 184 participants who experienced at least one CVD event during DCCT/EDIC, with a median follow-up of 27 years since randomisation. The number of CVD events for each 10 year interval of the DCCT/EDIC are presented in ESM Table 2. The predictive value of each covariate was described using the AUC at each year, as depicted in Fig. 2. Fig. 2a–d shows the predictive value of covariate values (e.g. SBP) at year 10 on subsequent CVD risk during years 10–20, adjusted only for age and diabetes duration (Model 1; diamonds); the AUC values are cumulative over the 10–20 year period, e.g. the value at 15 years is the AUC for years 10–15 and the value at 20 years being the AUC for years 10–20. Fig. 2 also shows the AUCs for a model with age and diabetes duration alone (Model 2; circles), HbA1c, age and diabetes duration (Model 3; squares), and the AUCs for a model with all covariates adjusted for HbA1c, age and diabetes duration (Model 4; triangles). Adjusted for age and diabetes duration, mean HbA1c (Model 3) performed similarly (similar AUC) to SBP (Model 1; Fig. 2a), but SBP was a stronger predictor of CVD (larger AUC) than the other CVD risk factors (Fig. 2a–d; squares vs diamonds). In additional models using covariate values at year 15 to predict CVD events over the subsequent 10 years (i.e., 15–25 years), there were negligible differences between the AUC of HbA1c and the other risk factors (data not shown).
AUC values for the updated mean SBP, LDLc levels, pulse rate or current triacylglycerol levels (Trigs), each adjusted for age and duration of diabetes (Model 1, diamonds) over (a–d) years 10–20 and (e–h) years 20–30 since enrolment. Also shown are the AUCs for models with age and diabetes duration alone (Model 2, circles), and age, diabetes duration and HbA1c (Model 3, squares), and the full model (Model 4, triangles). The data presented for the models with age and diabetes duration (Model 2, circles), age, diabetes duration and HbA1c (Model 3, squares), and the full model (Model 4, triangles) are identical in (a–d) and in (e–h)
Fig. 2e–h presents the AUC from models using the covariate values at year 20 to predict CVD events in years 20–30. Models with SBP, LDLc and triacyclglycerols (Model 1) had slightly higher AUCs than the model with HbA1c (Model 3; Fig. 2e–g, diamonds vs squares), while the AUC for pulse rate was similar to that of HbA1c (Fig. 2h, diamonds vs squares).
While the predictive ability of a model with only age and duration of diabetes (Model 2) remained approximately constant over time (Fig. 2, circles), adding mean HbA1c to the model (i.e., Model 3) was more predictive (higher AUC) in the 10–20 year model than in the 20–30-year model (Fig. 2, squares); the opposite was true for LDLc and triacylglycerols, but not for SBP and pulse rate (Fig. 2, diamonds). Moreover, the predictive ability of the full 20–30 year model (Model 4, described in ESM Table 1) was greater than that of the 10–20 year model (Fig. 2, triangles).
Possible mediation pathways may explain the change in the AUC of mean HbA1c over time. Among participants at risk at 10 years of follow-up, there were 89 participants with a CVD event in the subsequent 10–20 years, 97 with a CVD event between 15 and 25 years and 72 with a CVD event between 20 and 30 years. The total effect of mean HbA1c on the risk of CVD outcomes in the successive 10 year models (10–20, 11–21, etc.) was approximately constant over time (ESM Fig. 1), with a small dip at year 16. The average HbA1c coefficient value in these Aalen models was approximately 0.0033, indicating that for 1000 participants at risk, each 1 unit increase in mean HbA1c (e.g. from 7% to 8%, or a 10.9 mmol/mol increase) results in an average of 3.3 (=0.0033 × 1000) additional CVD events per year.
The decomposition of the total effect of the HbA1c over time into the direct and indirect effects mediated by each of the other covariates is presented in Table 1 and demonstrates that the indirect effect of each covariate (mediation pathway) increases over time while the direct effect of HbA1c decreases (Table 1 and ESM Fig. 1). For example, as noted above, in a model with HbA1c as the exposure and SBP as the mediator, a 1% (10.9 mmol/mol) higher HbA1c in 1000 individuals at risk at year 10 resulted in a total expected number (total effect) of 3.3 additional individuals with CVD events per year over years 10–20; of these 3.21 (97.3%) were attributable to the direct effect of HbA1c, and only 0.09 (2.7%) to the indirect pathway through SBP. The HbA1c values at year 15 had a higher indirect effect on the 15–25 year risk of CVD (0.51 events), and the values at year 20 had an even higher indirect effect on the 20–30 year risk (0.88 events). However, the total effect of the HbA1c values at years 10, 15 and 20 on risk of CVD in the subsequent 10 years remained approximately unchanged (3.3, 3.4, 3.4, respectively), and the direct effect declined modestly (3.2, 2.9, 2.6, respectively; Table 1).
Further, in mediation analyses, the SBP values at year 10 explained only 2.7% of the effect of the HbA1c values at year 10 on the 10–20 year CVD risk, but the values at 15 years explained 15.0% of the 15–25 year risk, and the values at 20 years explained 25.6% of the 20–30 year risk (Table 1).
Similar patterns were observed for the other three risk factors, with mediation proportion values over years 10–20 and 20–30 of 6.3% and 29.3% for pulse rate, 2.2% and 22.4% for triacylglycerols and 9.2% and 30.7% for LDLc (Table 1). Figure 3 depicts the increasing fraction of the HbA1c effect that is mediated by each of the other four factors over time, and ESM Table 3 presents a comparison of the mediation proportion values over time.
ESM Fig. 2 illustrates a model using both LDLc and SBP as mediators of the HbA1c effect on CVD but assuming no association of LDLc with SBP. The resulting mediation proportions (Table 2 and ESM Fig. 3) range from 8.5% over years 10–20 to 27.8% over years 20–30 for LDLc, and from 2.5% to 23.8%, respectively, for SBP.
The results of a mediation analysis that include all four variables (SBP, pulse rate, triacylglycerols and LDLc) as mediators (ESM Fig. 4) demonstrate that, while 85.8% of the HbA1c effect on CVD over years 10–20 was attributable to the direct HbA1c path (14.2% mediated by the other four covariates), this dropped to 58.3% over years 15–25 (42.7% mediation) and to 33.5% (66.5% mediation) over years 20–30 (Table 3, Fig. 4). Over years 20–30, LDLc was the most important mediator of the HbA1c effect (20.7% proportion explained), followed by SBP (18.7% proportion explained), pulse rate (15.2% proportion explained) and triacylglycerols (11.9% proportion explained).
Decomposition of the total effect of HbA1c on CVD risk into the direct and indirect effects mediated through multiple risk factors over time for the 10–20, 15–25 and 20–30 year models, using covariate values at 10, 15 and 20 years, respectively. Each shaded bar represents the proportion of the direct effect of HbA1c on CVD risk (black) and the indirect effects through SBP (dark grey), pulse rate (light grey), triacylglycerols (white) and LDLc (diagonal stripes). Data are presented in Table 3
Discussion
These analyses demonstrate that the effect of mean HbA1c on risk of CVD, when assessed in separate 10 year models starting at different points in time and adjusting for age and duration of diabetes, was largely constant over time. The coefficient was equivalent to an increase of approximately 3.3 CVD events per 1000 individuals per year for every 1% (10.9 mmol/mol) higher HbA1c. The 10 year predictive value (AUC) of HbA1c declined slightly from about 0.72 over the 10–20 year interval to 0.69 over the 20–30 year interval, whereas the AUC of more traditional CVD risk factors (SBP, pulse rate, triacylglycerols and LDLc) increased slightly. The 10 year risk associated with each covariate remained largely unchanged over time. However, the proportion of the total HbA1c effect on CVD risk that was mediated through its indirect effects on these CVD risk factors changed over time. Compared with the 14.2% of the HbA1c effect on the 10 year CVD risk mediated by its indirect effects on SBP, pulse rate, triacylglycerols or LDLc over 10–20 years of follow-up, 66.5% of the HbA1c effect on CVD risk was mediated by these other factors over 20–30 years of follow-up. Thus, while the total effect of HbA1c remained unchanged over time, the patterns of mediation changed substantially as the cohort aged (Fig. 3, Table 3).
The change in the indirect effect of HbA1c mediated through SBP, triacylglycerols, pulse rate and LDLc on CVD risk might be explained in several ways. The DCCT excluded individuals with high BP and hypercholesterolaemia at baseline. Thus, early in the study the only operant major risk factor was hyperglycaemia, which naturally predominated. It is also possible that hyperglycaemia has its major impact early in the course of CVD progression, e.g. in the early initiation of the atherosclerotic process. Conversely, SBP, triacylglycerols and LDLc may begin to affect CVD risk increasingly as individuals age, and the mean age of our cohort was only 27 at baseline. Indeed, the development of microalbuminuria and renal disease is known to worsen standard risk factors such as BP and lipids. In addition, there may be a cohort effect where participants with higher HbA1c values experience CVD events earlier in the study and are no longer at risk later during follow-up, e.g. at 20 years.
These mediation effects also apply to the net levels of SBP, LDLc and triacylglycerols as measured, regardless of whether or not the participant is receiving cardio-renal medical therapy (renin–angiotensin system [RAS] inhibitors, statins, etc.). It would be difficult to attempt to apply statistical modelling to estimate the mediation proportions in a population of individuals who were not receiving such medications. This is further complicated by the fact that medication use grew over time. At 10 years of follow-up, only 12.8% of participants were receiving a RAS inhibitor and 1.6% a statin/beta blocker. This usage increased to 58.3% and 51.8% at 20 years and 74.1% and 76.8% at 30 years, respectively. Further, using SBP as an example, anti-hypertensive medication use would translate into a lower indirect effect of HbA1c on SBP and, subsequently, on CVD, and therefore a lower mediation proportion for the effect of SBP on CVD. As medication use increased, the mediation proportion for SBP is, if anything, an underestimate of the value without medication use.
These results have clinical implications and provide further insight into possible mechanisms leading to CVD in type 1 diabetes. Over 20–30 years of follow-up (27–37 years of diabetes duration), LDLc mediated 31% of the effect of HbA1c. Of the average additional 3.4 individuals with a CVD event per year associated with each 1% (10.9 mmol/mol) increase in HbA1c, ~1 excess CVD event per year is therefore attributable to LDLc (3.4 × 0.31 = 1.05 CVD events per year). This fraction of increased risk associated with 1% (10.9 mmol/mol) higher HbA1c might be eliminated by treatments aimed to lower LDLc. Our findings further reinforce the value of aggressive glycaemic management early in the course of diabetes, followed by aggressive management of the other CVD risk factors as they become increasingly operant.
Regarding possible mechanisms, the year-to-year total effect (direct and indirect) of HbA1c on the subsequent 10-year risk of CVD is relatively constant over time, suggesting aggressive glycaemic management has the potential to reduce the CVD risk throughout the course of diabetes. At the same time, the fraction of the HbA1c effect on CVD risk that is mediated by its effects on these other covariates increases substantially over time. This finding suggests that aggressive management of traditional non-glycaemic CVD risk factors may further reduce the risk of CVD in those with longstanding diabetes and long-term glycaemic exposure above the target levels.
Abbreviations
- CVD:
-
Cardiovascular disease
- EDIC:
-
Epidemiology of Diabetes Interventions and Complications
- LDLc:
-
LDL-cholesterol
- MACE:
-
Major atherosclerotic cardiovascular events
- NIDDK :
-
National Institute of Diabetes and Digestive and Kidney Diseases
- SBP:
-
Systolic BP
References
Orchard TJ, Costacou T, Kretowski A, Nesto RW (2006) Type 1 diabetes and coronary artery disease. Diabetes Care 29:2528–2538
Livingstone SJ, Looker HC, Hothersall EJ et al (2012) Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 9:e1001321
Nathan DM, Cleary PA, Backlund JY et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653
Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group (2016) Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 39:686–693
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group (2016) Risk factors for cardiovascular disease in type 1 diabetes. Diabetes 65:1370–1379
Paterson AD, Rutledge BN, Cleary PA, Lachin JM, Crow RS (2007) Diabetes control and complications trial/epidemiology of diabetes interventions and complications research group: the effect of intensive diabetes treatment on resting heart rate in type 1 diabetes: the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 30:2107–2112
de Boer IH, Rue TC, Cleary PA et al (2011) Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the diabetes control and complications trial/epidemiology of diabetes interventions and complications cohort. Arch Intern Med 171:412–420
The DCCT/EDIC Research Group (1999) Epidemiology of diabetes interventions and complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the diabetes control and complications trial cohort. Diabetes Care 22:99–111
The DCCT Research Group (1986) The diabetes control and complications trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 35:530–545
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Lachin JM, Orchard TJ, Nathan DM (2014) DCCT/EDIC research group: update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 37:39–43
Martinussen T, Scheike TH (2006) Dynamic regression models for survival data. Springer, New York
D'Agostino RBS, Vasan RS, Pencina MJ et al (2008) General cardiovascular risk profile for use in primary care: the framingham heart study. Circulation 117:743–753
Goff DC Jr, Lloyd-Jones DM, Bennett G et al (2014) American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 63:2935–2959
Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves. Biometrics 61:92–105
Lange T, Hansen JV (2011) Direct and indirect effects in a survival context. Epidemiology 22:575–581
Acknowledgements
A complete list of participants in the DCCT/EDIC Research Group is presented in the ESM published online for this article.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Data availability
All DCCT/EDIC data files are available at the NIDDK Repository (www.niddkrepository.org/studies/edic).
Funding
The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993, 2012–2017) and contracts (1982–2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007) and Clinical Translational Science Center Program (2006-present), Bethesda, MD, USA. The following industry contributors had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA, USA), Animas (Westchester, PA, USA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY, USA), Becton Dickinson (Franklin Lakes, NJ, USA), Eli Lilly (Indianapolis, IN, USA), Extend Nutrition (St Louis, MO, USA), Insulet Corporation (Bedford, MA, USA), Lifescan (Milpitas, CA, USA), Medtronic Diabetes (Minneapolis, MN, USA), Nipro Home Diagnostics (Ft Lauderdale, FL, USA), Nova Diabetes Care (Billerica, MA, USA), Omron (Shelton, CT, USA), Perrigo Diabetes Care (Allegan, MI, USA), Roche Diabetes Care (Indianapolis, IN, USA), and Sanofi-Aventis (Bridgewater, NJ, USA). The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
IB and JML wrote the initial draft of the manuscript. IB and BHB conducted statistical analyses. All authors contributed to the study design and the interpretation of the data, revised the manuscript for important intellectual content and approved the final version. IB is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
The complete list of the DCCT/EDIC Research Group is provided in the electronic supplementary material (ESM).
Electronic supplementary material
ESM
(PDF 259 kb)
Rights and permissions
About this article
Cite this article
Bebu, I., Braffett, B.H., Pop-Busui, R. et al. The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60, 2084–2091 (2017). https://doi.org/10.1007/s00125-017-4374-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-017-4374-4