Abstract
Aims/hypothesis
We aimed to investigate the potential mechanisms involved in the compromised adipogenesis of visceral (VAT) vs subcutaneous adipose tissue (SAT) using comparative metabolomics. Based on the differentially identified metabolites, we focused on the relationship between the active form of vitamin B6 (pyridoxal 5-phosphate [PLP]), known to be generated through pyridoxal kinase (PDXK), and adipogenesis.
Methods
Non-targeted metabolomics analyses were performed in paired VAT and SAT (n = 14, discovery cohort). PDXK gene expression was evaluated in two validation cohorts of paired SAT and VAT samples in relation to obesity status and insulin sensitivity, and mechanistically after weight loss in vivo and in 3T3-L1 cells in vitro.
Results
Comparative metabolomics showed that PLP was significantly decreased in VAT vs SAT. Concordantly, PDXK mRNA levels were significantly decreased in VAT vs SAT, specifically in adipocytes. The decrease was specially marked in obese individuals. PDXK mRNA levels showed a strong association with adipogenic, lipid-droplet-related and lipogenic genes. At a functional level, systemic insulin sensitivity positively associated with PDXK expression, and surgically-induced weight loss (improving insulin sensitivity) led to increased SAT PDXK mRNA levels in parallel with adipogenic genes. In human pre-adipocytes, PDXK mRNA levels increased during adipocyte differentiation and after administration of peroxisome proliferator-activated receptor-γ agonists, and decreased under inflammatory stimuli. Mechanistic studies in 3T3-L1 cells showed that PLP administration resulted in increased adipogenic mRNA markers during early adipogenesis, whereas the PLP antagonist 4-deoxypyridoxine exerted opposite effects.
Conclusions/interpretation
Overall, these results support the notion that in situ production of PLP is required for physiological adipogenesis.
Similar content being viewed by others
Introduction
Aside from evident differences in anatomical location, adipose tissue depots present in subcutaneous and omental sites are functionally and pathophysiologically different. Obesity-related complications are mainly associated with the presence and amount of omental adipose tissue, leading to the assumption of a distinct deleterious role, as demonstrated in population studies [1]. The reasons for such differences from subcutaneous tissue may arise from differential accumulation of inflammatory cells and a different lipid composition [2]. Omental adipose tissue-derived metabolites, through the anatomical connection mediated by the portal vein, could reach hepatocytes and/or interact with absorbed nutrients [3]. Similarly, metabolic activity at each location differs markedly in sensitivity to endocrine regulators of lipolysis, such as catecholamines and insulin [3]. The differences may be related to obesity-associated growth of adipose tissue, as lipidomic signatures specific to the omental location only arise in obese individuals [2]. The use of systems biology-based approaches (such as genomics, transcriptomics, proteomics and lipidomics) permits a molecular fingerprinting of these tissues. Transcriptomics revealed differentially expressed genes belonging to adipogenesis and lipid metabolism ontogenies both in vivo [4–6] and in cells in vitro [7]. This fits with a differential pattern of protein expression, with location-specific traits in proteins involved in lipid oxidation–reduction and transport [8].
In contrast to genes and proteins, metabolites provide the most direct evidence of biochemical changes and they can be easily correlated with a given phenotype. Metabolomics based on gas chromatography techniques has been previously tested in adipose tissue from differential locations [9]. Although it is robust, this technique shows a relatively low number of potential candidates when compared with liquid chromatography coupled with high-precision mass spectrometry.
In the present work we demonstrate the existence of specific metabolomic signatures of human omental and subcutaneous adipose tissue (SAT), mainly attributed to selective enrichment in important bioactive compounds. Pyridoxal 5-phosphate (PLP) was one of the most differentially detected metabolites, being significantly decreased in visceral adipose tissue (VAT) vs SAT. Since subsequent analyses showed that PDXK is highly expressed in human adipose tissue, we explored the possible link between PDXK and adipogenesis in human adipose tissue, by employing both a 3T3-L1 mouse cell line and human adipocytes, confirming the importance of PLP and PDXK in adipocyte differentiation.
Methods
Recruitment of donors for adipose tissue samples
Cross-sectional studies
In the first and second cohort, a group of 196 tissues (101 VAT and 95 SAT) (cohort 1) from participants with normal body weight and different degrees of obesity, with BMI 20–68 kg/m2, were analysed (for characteristics of cohort, see Table 1). This cohort included 70 paired SAT and VAT samples (eight non-obese and 62 obese participants). In a second cohort of morbidly obese (BMI >35 kg/m2) individuals with different degrees of insulin activity (measured using hyperinsulinaemic–euglycaemic clamp [6], as detailed in the ESM Methods), 44 paired SAT and VAT samples (cohort 2) were studied (Table 1). The participants were recruited at the Endocrinology Service of the Hospital of Girona Dr Josep Trueta. All were of European descent and reported that their body weight had been stable for at least 3 months before the study. Participants were studied in the post-absorptive state. BMI was calculated as weight (in kg) divided by height (in m) squared. They had no systemic disease other than obesity and all were free of any infections in the month leading up to the study. Liver diseases (specifically tumours and hepatitis C infection) and thyroid dysfunction were specifically excluded by biochemical work-up. All participants gave written informed consent, validated and approved by the Ethics committee of the Hospital of Girona Dr Josep Trueta, after the purpose of the study was explained to them.
Samples were obtained from SAT and VAT depots during elective surgical procedures (cholecystectomy, surgery of abdominal hernia and gastric bypass surgery). Samples of adipose tissue were immediately transported to the laboratory (5–10 min). The handling of tissue was carried out under strictly aseptic conditions. Adipose tissue samples were washed in PBS, cut with forceps and scalpel into small pieces (100 mg) and immediately flash-frozen in liquid nitrogen before being stored at −80°C. Adipocyte and stromal vascular fraction (SVF) cells were isolated from eight SAT and eight VAT non-frozen samples. These samples were washed three or four times with PBS and suspended in an equal volume of PBS supplemented with 1% vol./vol. penicillin–streptomycin and 0.1% (wt/vol.) collagenase type I pre-warmed to 37°C. The tissue was placed in a shaking water bath at 37°C with continuous agitation for 60 min and centrifuged for 5 min at 300–500 g at room temperature. The supernatant fraction, containing mature adipocytes, was collected. The pellet was identified as the SVF. Isolated mature adipocytes and SVF were stored at −80°C for gene expression analysis.
Intervention study
The third cohort consisted of 18 obese (BMI 43.67 ± 4.6 kg/m2, age 49.1 ± 7.6 years [mean ± SD]) individuals of European descent, who underwent bariatric surgery (Roux-en-Y gastric bypass [RYGB]) at the Hospital of Girona Dr Josep Trueta and were part of an ongoing study [6]. Inclusion criteria were age 30–60 years, BMI ≥ 35 kg/m2 and ability to understand the study protocol. Exclusion criteria were use of medications able to interfere with insulin’s action and history of a chronic systemic disease. Samples from the SAT depot were obtained during bariatric surgery. Postoperative samples of SAT were obtained by subcutaneous biopsy at the mesogastric level 2 years after surgery. Fasting blood samples were obtained on the same day as the biopsy. All participants gave written informed consent, validated and approved by the ethical committee of the Hospital of Girona Dr Josep Trueta, after the purpose of the study was explained to them.
Analytical methods
Serum glucose concentrations were measured in duplicate by the glucose oxidase method using a Beckman glucose analyser II (Beckman Instruments, Brea, CA, USA). A Roche Hitachi Cobas c711 instrument (Roche, Barcelona, Spain) was used to determine HDL-cholesterol and total serum triacylglycerols. HDL-cholesterol was quantified by a homogeneous enzymatic colorimetric assay through the cholesterol esterase–cholesterol oxidase–peroxidase reaction (Cobas HDLC3). Serum fasting triacylglycerols were measured by an enzymatic, colorimetric method with glycerol phosphate oxidase and peroxidase (Cobas TRIGL).
Non-targeted metabolomics
Metabolites were analysed in the discovery cohort in 14 paired VAT and SAT samples (four men and ten women) with methanol according to previously described methods [2]. Briefly, 20 volumes (vol./wt) of cold methanol (containing 1 μmol/l butylated hydroxytoluene) were added to 35–50 mg of adipose tissue and homogenised with a Polytron device at 4°C. Then, 20 volumes of lysis buffer (consisting of 180 mmol/l KCl, 5 mmol/l EDTA and 1 mmol/l diethylenetriaminepentaacetic acid, pH 7.3) were added and homogenates were incubated at −20°C for 1 h and centrifuged at 12,000 g for 3 min. The supernatant fractions were recovered, evaporated using a Speed Vac (Thermo Fisher Scientific, Barcelona, Spain) and resuspended in 0.4% vol./vol. acetic acid and methanol (1 : 1 vol./vol.).
For the metabolomics studies, an Agilent 1290 LC system coupled to an electrospray ionisation quadrupole time-of-flight (ESI-Q-TOF) MS/MS 6520 instrument (Agilent Technologies, Barcelona, Spain) was used. In all cases, 2 μl of extracted sample was applied onto a reverse-phase column (Zorbax SB-Aq, 1.8 μm 2.1 × 50 mm; Agilent Technologies) equipped with a precolumn (Zorba-SB-C8 Rapid Resolution Cartridge, 2.1 × 30 mm 3.5 μm; Agilent Technologies) with a column temperature of 60°C. The flow rate was 0.6 ml/min. Solvent A was composed of water containing 0.2% vol./vol. acetic acid and solvent B was composed of methanol and 0.2% acetic acid. The gradient started at 2% vol./vol. B and increased to 98% vol./vol. B in 13 min, and then held at 98% B for 6 min. Post-time was established in 5 min.
Data were collected in positive and negative electrospray mode TOF operated in full-scan mode at 50–1,600 m/z in an extended dynamic range (2 GHz), using N2 as the nebuliser gas (10 l/min, 350°C). The capillary voltage was 4,000 V with a scan rate of 1.5 scan/s. The ESI source used a separate nebuliser for the continuous, low-level (10 l/min) introduction of reference mass compounds: 121.050873, 922.009798 (positive ion mode) and 119.036320, 966.000725 (negative ion mode), which were used for continuous, online mass calibration. The MassHunter Data Analysis Software (Agilent Technologies) was used to collect the results and the MassHunter Qualitative Analysis Software (Agilent Technologies) was used to obtain the molecular features of the samples, representing different, co-migrating ionic species of a given molecular entity using the Molecular Feature Extractor algorithm (Agilent Technologies), as described [10, 11]. Finally, the MassHunter Mass Profiler Professional Software (Agilent Technologies) was used to perform a non-targeted metabolomic analysis of the extracted features. We selected samples with a minimum absolute abundance of 5,000 counts and with a minimum of two ions. Multiple charge states were not considered. Compounds from different samples were aligned using a retention time (RT) window of 0.1% ± 0.15 min and a mass window of 5.0 ppm ± 2.0 mDa. Only common features (found in at least 75% of the samples at the same condition) were analysed, correcting for individual bias. Data for principal component analysis (PCA) and partial least-square discriminant analysis (PLS-DA) were obtained using this software. The masses with significant differences (paired t test, p < 0.05; Benjamini Hochberg correction for false discovery rate) in abundance were searched against METLIN Metabolite PCD/PCDL database (Agilent Technologies). This database allows the identification of metabolites based on isotope distribution, exact mass and identical chromatographic conditions with authentic standards. Pathway analysis was performed using Metaboanalyst platform [12] and resulting pathways were included in the ConsensusPathDB Interaction database [13].
RNA expression
RNA purification, gene expression procedures and analyses were performed, as detailed in electronic supplementary material (ESM) Methods and previously described [6, 14, 15].
Differentiation of human pre-adipocytes
Isolated human subcutaneous and visceral pre-adipocytes (Zen-Bio, Research Triangle Park, NC, USA) were cultured and differentiated as detailed in ESM Methods.
TNFα (100 ng/ml) and macrophage-conditioned medium (MCM, 5% vol./vol.) from THP-1 cells previously treated with lipopolysaccharide (10 ng/ml, 24 h) were added during both SAT and VAT adipocyte differentiation. Otherwise, after adipocyte differentiation (on day 14), SAT fully differentiated adipocytes were incubated with fresh medium (control) or fresh medium containing rosiglitazone (0.1 and 1 μmol/l). After 48 h cells were harvested and pellets and supernatant fractions were stored at −80°C.
All in vitro experiments using human cells were performed in triplicate and experimenters were blind to outcome assessment.
Mouse 3T3-L1 cell differentiation and mechanistic studies
The embryonic fibroblast mouse cell line 3T3-L1 (American Type Culture Collection; LGC Standards, Barcelona, Spain) was cultured in DMEM containing 20 mmol/l glucose, 10% vol./vol. calf bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Mycoplasma contamination was not detected in this cell line. During 3T3-L1 adipocyte differentiation, calf bovine serum was replaced with fetal bovine serum. Two days after confluence, insulin (5 μg/ml), dexamethasone (0.5 μmol/l) and isobutylmethylxanthine (0.5 mmol/l) mixture was added for 2 days, followed by 5 days with insulin (5 μg/ml) alone, which was replaced on day 5. Cells were then considered mature adipocytes, harvested on day 7 and stored at −80°C for RNA purification. To demonstrate the functional role of PLP, 4-deoxypyridoxine (4-DP; 5 and 50 μmol/l) and PLP (0.5, 5 and 50 μmol/l) administration was performed during adipocyte differentiation. These experiments were performed in triplicate and experimenters were blind to outcome assessment.
Statistical analyses
Statistical analyses were performed using SPSS 12.0 software. Unless otherwise stated, descriptive results of continuous variables are expressed as mean ± SD for Gaussian variables or median and interquartile range. Variables that did not fulfil normal distribution were log transformed to improve symmetry for subsequent analyses. The relationship between variables was analysed by simple correlation (Pearson’s test and Spearman’s test) and multiple regression analyses in a stepwise manner. For non-targeted metabolomics, a paired t test was used, with Benjamini–Hochberg correction for false discovery rate. Otherwise, one-factor ANOVA with post hoc Bonferroni test, paired t test and unpaired t test were used to compare PDXK gene expression according to obesity. A nonparametric test (Mann–Whitney test) was used to analyse in vitro experiments. In all cases, the level of statistical significance was set at p < 0.05.
Results
Non-targeted metabolomics discloses a location-specific metabolomics profile
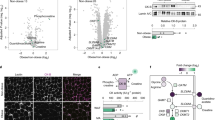
Non-targeted metabolomics showed that many molecular features (51 downregulated and 513 upregulated in VAT vs SAT, in positive ionisation; 37 downregulated and 98 upregulated in VAT vs SAT in negative ionisation) differed significantly in their concentrations (ESM Dataset 1), as demonstrated by hierarchical clustering, heatmaps and both unsupervised (PCA) and supervised multivariate analyses (PLS-DA) with an accuracy exceeding 95% (Fig. 1a, b). To reinforce this, we employed alternative classification procedures, such as random forest analyses, which demonstrated an error rate below 18% (Fig. 1c). Furthermore, the recursive supported vector machine scheme showed a model with a 7.1% error rate with only five molecular species (Fig. 1d). Multivariate classification procedures indicate a specific profile of metabolites in SAT vs VAT. As indicate both by Random forest analyses (Fig. 1c) and recursive supported vector machine classification (Fig. 1d), a relatively low error of classification could be obtained with few compounds.
(a, b) Location-specific metabolomics profiling discloses PLP metabolism as a differential trait with both hierarchical clustering of samples (Euclidean distances, Ward clustering algorithm), using the top 50 differentially expressed molecules selected by random forest algorithm (colour range bar shows the concentration of these differential molecules [log range for autoscaled data]) (a) and multivariate analyses (PCA and PLS-DA) (b), with red spheres indicating VAT samples and blue spheres SAT samples. (c, d) Multivariate classification procedures performed included random forest analyses (c) and recursive supported vector machine (SVM) classification (d). For random forest classification, an overall classification error of 0.17 (in green) was obtained (0.14 for SAT, in blue; 0.21 for VAT, in red)
Based on accurate mass, isotope distribution and retention time equality with authentic standards, nine metabolites were identified (Table 2). Pathway analyses (ESM Table 1 and ESM Fig. 1) clustered these metabolites across two major networks: one comprising adipocytokine and peroxisome proliferator-activated receptor (PPAR) signalling and the other including vitamin metabolism. Based on very early data [16–19] we focused on vitamin B6 metabolism: the levels of vitamin B6 active metabolite PLP were significantly decreased in VAT vs SAT (Fig. 2a). Consistently, in the discovery and validation cohorts, mRNA levels of PDXK, encoding the enzyme needed for PLP synthesis, were also detected in human adipose tissue, being significantly downregulated in VAT vs SAT (Fig. 2b, c). Interestingly, PDXK gene expression was positively correlated with PLP levels in SAT (Fig. 2d), but not in VAT (Fig. 2e). PDXK mRNA was significantly enriched in the adipocyte fraction of adipose tissue (Fig. 2f).
(a–c) PLP levels were diminished in VAT (n = 14) (a), shown in adjusted mass spectrometry (MS) counts, and this was associated with diminished expression of PDXK in VAT in two different cohorts (b, c): cohort 1 (n = 70) (b) and cohort 2 (n = 44) (c). ***p < 0.001 compared with SAT. (d, e) Furthermore, SAT PDXK (r = 0.58, p = 0.04) (d) but not VAT (r = −0.07, p = 0.8) (e) expression correlates with PLP concentrations. Although PLP levels were analysed in all samples, mRNA was available in 12 of these 14 paired samples. Furthermore, PLP levels were very low in one of the VAT samples. (f) PDXK gene expression in adipose tissue fractions in SAT (dark grey bars) and VAT (light grey bars). **p < 0.01 compared with SVF; † p < 0.05 compared with subcutaneous adipocytes. (g, h) PDXK expression during subcutaneous (g) and visceral (h) adipocyte differentiation. *p < 0.05 and **p < 0.01 compared with day 0. (i) Effect of inflammatory stimuli on PDXK gene expression during subcutaneous adipocyte differentiation. **p < 0.01 compared with differentiated control (Diff). (j) Effect of exposure to a PPAR agonist (rosiglitazone, Rosigl; 0.1 and 1 μmol/l) on PDXK gene expression in mature adipocytes. *p < 0.05 and **p < 0.01 compared with differentiated control (Diff). (k) Effect of PLP (0.5, 5 and 50 μmol/l) administration on adipogenic gene expression, such as ADIPOQ (white bars) and GLUT4 (dark grey bars). (l) Effect of 4DP (5 and 50 μmol/l) administration on expression of adipogenesis transcriptional markers, such as ADIPOQ, PPARG, GLUT4, PLIN1, and the inflammatory IL6 gene. *p < 0.05 and **p < 0.01 compared with differentiated control (Diff)
PDXK is implicated in adipogenic differentiation in human adipose tissue
To test whether PDXK could be implied in the adipogenic program of human adipose tissue, we compared its expression with accepted markers of adipogenesis in three independent cohorts. In the discovery cohort (Table 1), PDXK gene expression was significantly associated with expression of adipogenic genes, including PPARG, lipid-droplet-related (CIDEC, PLIN1) and lipogenesis-related (FASN, ACACA) genes both in SAT and in VAT (Table 3). Otherwise, PDXK gene expression was negatively associated with TMEM26 mRNA levels (a specific marker of beige adipocytes) and no significant relationships were found with the expression of inflammatory gene markers (LEP, IL6, TNF and CD68) (Table 3). VAT PDXK gene expression was especially decreased in obese participants (Table 1) and negatively associated with BMI (Table 3). These results were confirmed in a validation cohort (Table 1) in which PDXK gene expression was negatively correlated with BMI and TMEM26 in VAT, and positively associated with adipogenic (PPARG, ADIPOQ, GLUT4 [also known as SLC2A4]), lipid-droplet-related (CIDEC, PLIN1) and lipogenic (DGAT1) gene expression in both VAT and SAT (Table 4).
To confirm these associations in paired VAT and SAT, all paired samples from both discovery and validation cohorts (n = 114) were analysed together. Interestingly, in both VAT and SAT, PDXK gene expression was positively correlated with the expression of adipogenic markers, including ADIPOQ (r = 0.31, p = 0.001 in VAT and r = 0.33, p = 0.001 in SAT), GLUT4 (r = 0.44, p < 0.0001 in VAT and r = 0.50, p < 0.0001 in SAT), PLIN1 (r = 0.40, p < 0.0001 in VAT and r = 0.43, p < 0.0001 in SAT) and CIDEC (r = 0.46, p < 0.0001 in VAT and r = 0.51, p < 0.0001 in SAT), and negatively with TMEM26 (r = −0.29, p = 0.004 in VAT and r = −0.28, p = 0.004 in SAT).
To further study the potential importance of PLP in adipogenesis at a functional level, SAT PDXK gene expression was studied according to systemic insulin sensitivity (measured using hyperinsulinaemic–euglycaemic clamp), confirming a positive association (Table 4). Bariatric surgery-induced weight loss, which resulted in a significant increase in the expression of adipogenic genes [6], also led to increased PDXK mRNA levels gene expression (Table 5). Of note, the change in PDXK gene expression was positively correlated with the change in adipogenic gene expression (FASN [r = 0.67, p = 0.004], PLIN1 [r = 0.71, p = 0.002], DGAT1 [r = 0.74, p = 0.001], IRS1 [r = 0.58, p = 0.01], PPARG [r = 0.47, p = 0.06] and ADIPOQ [r = 0.44, p = 0.09]).
PDXK is required for full adipogenic differentiation
PDXK gene expression increased during adipocyte differentiation of both VAT and SAT adipocytes, with the increase being significantly attenuated in VAT vs SAT adipocyte differentiation (Fig. 2g, h). This is in line with decreased PLP values in VAT in vivo, suggesting a differential response towards the adipogenic effects of vitamin B6 according to anatomical location. Inflammatory stimuli resulted in decreased PDXK gene expression (Fig. 2i), whereas PPARγ agonists led to increased PDXK mRNA levels in a dose-dependent manner (Fig. 2j). In line with data from human samples, and reinforcing the importance of PLP in adipogenesis, PLP administration (0.5, 5 and 50 μmol/l) during early adipogenesis (day 0–2) resulted in a dose-dependent increase in adipogenic gene expression markers (ADIPOQ and GLUT4) (Fig. 2k). On the contrary, administration of 4DP, a PLP antagonist (50 μmol/l), led to a significant reduction in the expression of adipogenic genes (ADIPOQ, PPARG, GLUT4 and PLIN1) and increased expression of the proinflammatory gene IL6 (Fig. 2l).
Discussion
Pyridoxal (pyridoxine, vitamin B6) kinase is a cytosolic enzyme (EC:2.7.1.35) that phosphorylates vitamin B6, an essential step for the conversion of vitamin B6 to PLP (the active form of vitamin B6) [20]. PLP acts as a cofactor for more than 140 enzymes, which catalyse multiple biochemical reactions and participate in a wide range of metabolic and developmental processes including amino acid, fatty acid and neurotransmitter metabolism [20, 21]. Furthermore, PLP attenuates the production of oxygen reactive species [22] and the formation of advanced glycation end-products [23]. In fact, vitamin B has been used in the treatment of diabetic complications [24], though its use has to be viewed with caution [25].
To the best of our knowledge, this is the first study to demonstrate substantial PLP levels and PDXK gene expression in human adipose tissue, with evident differences between pathogenically relevant distinct anatomical locations. The link between PDXK and adipogenesis was supported by several lines of evidence. First, in line with adipogenic markers [6, 26], PLP levels and PDXK gene expression was decreased in VAT vs SAT and adipocytes, with the associated differences in their adipogenic potential. Second, PDXK gene expression was significantly associated with the expression of adipogenic genes in the discovery and validation cohort. Similar to lipogenic and adipogenic genes [6, 26], VAT PDXK mRNA levels were negatively correlated with BMI. Third, bariatric surgery-induced weight loss led to increased PDXK gene expression in positive correlation with adipogenic genes. Fourth, similar to adipogenic genes, PDXK mRNA levels increased during human adipocyte differentiation. Inflammatory conditions attenuated the increase of PDXK mRNA levels during adipocyte differentiation, whereas a PPARγ agonist enhanced PDXK mRNA levels. Finally, the inhibition of PDXK activity (using 4-DP) attenuated adipogenic gene expression during adipocyte differentiation, whereas PLP administration in the early stage of adipocyte differentiation had the opposite effect.
Altogether these data support the notion that in situ production of PLP is required for physiological adipogenesis. Previous knowledge was based on the effects of vitamin B6 per se. A vitamin B6-deficient diet resulted in a significant reduction in adipose tissue and lipogenesis in rat models [16–18]. Vitamin B6 administration increased intracellular lipid accumulation in 3T3-L1 adipocytes [27] and reduced macrophage infiltration and adipose tissue inflammation in mice [19, 28].
Current findings show that vitamin B6 needs to be transformed to PLP to show these effects. In fact, PLP induced PPARγ target genes in 3T3-L1 and NHI3T3 cells, stimulating PPARγ transactivation [27]. PPARγ binding sites are co-localised in the promoters of most genes, which change during adipogenesis [29–31]. In the current study, PPAR signalling appeared as a relevant node in the metabolomic network analyses differentiating SAT and VAT. However, other hypotheses on the role of PLP in adipogenesis cannot be dismissed. Huq et al [32] demonstrated that mouse nuclear receptor interacting protein 140 (RIP140) can be modified by PLP conjugation and, in consequence, RIP140 transcriptional co-repressive activity and its physiological function in adipocyte differentiation are enhanced. RIP140 participates in adipocyte fat accumulation and lipogenesis and in the maintenance of white adipocyte phenotype [33, 34]. Otherwise, PLP is a cofactor required for the optimal activity of both mitochondrial and cytoplasmic isoenzymes of serine hydroxymethyltransferase (SHMT1) and (SHMT2) [35, 36]. These enzymes are essential in folate-mediated one-carbon metabolism, which is compartmentalised in the mitochondria and cytoplasm and is known to have an important role in DNA methylation, synthesis and repair [36, 37]. Altered DNA methylation pattern has been recently associated with adipose tissue dysfunction in patients with type 2 diabetes [38]. Current data showing a link between PLP levels and adipogenesis imply that PLP has a possible role in adipose tissue DNA methylation through folate-mediated one-carbon metabolism. In fact, deficiency of vitamin B6 and B12 is known to be associated with altered methylation pattern and decreased DNA stability through inhibition of DNA synthesis and repair [39]. PLP has an important role in the maintenance of genome integrity [40]. Further studies will be required to explore this hypothesis.
Another potentially important finding of the present study was the positive association between SAT PDXK gene expression and insulin sensitivity. Furthermore, PDXK mRNA levels after bariatric surgery-induced weight loss ran in parallel with increased systemic insulin sensitivity in association with the expression of PPARγ target genes [27, 41–43]. In line with these results, plasma PLP levels were found to be negatively associated with insulin resistance-associated variables (such as C-reactive protein concentration, fasting glucose and HbA1c) in a previous study [44], whereas increased plasma PLP levels correlated with blood glucose reduction in surgically ill patients [45].
In conclusion, the present study suggests a novel role of adipose tissue PDXK activity and PLP levels in adipogenesis and systemic insulin sensitivity, with anatomical differences between SAT and VAT.
Abbreviations
- 4-DP:
-
4-Deoxypyridoxine
- MCM:
-
Macrophage-conditioned medium
- PCA:
-
Principal component analysis
- PDXK:
-
Pyridoxal kinase
- PLP:
-
Pyridoxal 5′-phosphate
- PLS-DA:
-
Partial least-square discriminant analysis
- PPAR:
-
Peroxisome proliferator-activated receptor
- RIP140:
-
Receptor interacting protein 140
- SAT:
-
Subcutaneous adipose tissue
- SVF:
-
Stromal vascular fraction
- VAT:
-
Visceral adipose tissue
References
Fox CS, Massaro JM, Hoffmann U et al (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48
Jové M, Moreno-Navarrete JM, Pamplona R, Ricart W, Portero-Otín M, Fernández-Real JM (2014) Human omental and subcutaneous adipose tissue exhibit specific lipidomic signatures. FASEB J 28:1071–1081
van Harmelen V, Dicker A, Rydén M et al (2002) Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes 51:2029–2036
Gesta S, Tseng YH, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131:242–256
MacLaren R, Cui W, Simard S, Cianflone K (2008) Influence of obesity and insulin sensitivity on insulin signaling genes in human omental and subcutaneous adipose tissue. J Lipid Res 49:308–323
Ortega FJ, Mercader JM, Moreno-Navarrete JM et al (2015) Surgery-induced weight loss is associated with the downregulation of genes targeted by microRNAs in adipose tissue. J Clin Endocrinol Metab 100:E1467–E1476
Perrini S, Laviola L, Cignarelli A et al (2008) Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia 51:155–164
Pérez-Pérez R, Ortega-Delgado FJ, García-Santos E et al (2009) Differential proteomics of omental and subcutaneous adipose tissue reflects their unalike biochemical and metabolic properties. J Proteome Res 8:1682–1693
Hanzu FA, Vinaixa M, Papageorgiou A et al (2014) Obesity rather than regional fat depots marks the metabolomic pattern of adipose tissue: an untargeted metabolomic approach. Obesity (Silver Spring) 22:698–704
Sana TR, Roark JC, Li X, Waddell K, Fischer SM (2008) Molecular formula and METLIN Personal Metabolite Database matching applied to the identification of compounds generated by LC/TOF-MS. J Biomol Tech 19:258–266
Jové M, Serrano JC, Ortega N et al (2011) Multicompartmental LC-Q-TOF-based metabonomics as an exploratory tool to identify novel pathways affected by polyphenol-rich diets in mice. J Proteome Res 10:3501–3512
Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS (2012) MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res 40:W127–W133
Kamburov A, Stelzl U, Lehrach H, Herwig R (2013) The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res 39:D712–D717
Moreno-Navarrete JM, Serrano M, Sabater M et al (2013) Study of lactoferrin gene expression in human and mouse adipose tissue, human pre-adipocytes and mouse 3T3-L1 fibroblasts. Association with adipogenic and inflammatory markers. J Nutr Biochem 24:1266–1275
Neville MJ, Collins JM, Gloyn AL, McCarthy MI, Karpe F (2011) Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity (Silver Spring) 19:888–892
Huber AM, Gershoff SN, Hegsted DM (1964) Carbohydrate and fat metabolism and response to insulin in vitamin B6-deficient rats. J Nutr 82:371–378
Ribaya JD, Gershoff SN (1977) Effects of vitamin B6 deficiency on liver, kidney, and adipose tissue enzymes associated with carbohydrate and lipid metabolism, and on glucose uptake by rat epididymal adipose tissue. J Nutr 107:443–452
Radhakrishnamurty R, Angel JF, Sabry ZI (1968) Response of lipogenesis to repletion in the pyridoxine-deficient rat. J Nutr 95:341–348
Sanada Y, Kumoto T, Suehiro H et al (2013) RASSF6 expression in adipocytes is down-regulated by interaction with macrophages. PLoS One 8, e61931
Hellmann H, Mooney S (2010) Vitamin B6: a molecule for human health? Molecules 15:442–459
Amadasi A, Bertoldi M, Contestabile R et al (2007) Pyridoxal 5'-phosphate enzymes as targets for therapeutic agents. Curr Med Chem 14:1291–1324
Ehrenshaft M, Bilski P, Li MY, Chignell CF, Daub ME (1999) A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc Natl Acad Sci U S A 96:9374–9378
Booth AA, Khalifah RG, Todd P, Hudson BG (1997) In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J Biol Chem 272:5430–5437
House AA, Eliasziw M, Cattran DC et al (2010) Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA 303:1603–1609
Thornalley PJ, Rabbani N (2010) Therapy: vitamin B6, B9 and B12 in diabetic nephropathy—beware. Nat Rev Endocrinol 6:477–478
Ortega FJ, Mayas D, Moreno-Navarrete JM et al (2010) The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 18:13–20
Yanaka N, Kanda M, Toya K, Suehiro H, Kato N (2011) Vitamin B6 regulates mRNA expression of peroxisome proliferator-activated receptor-γ target genes. Exp Ther Med 2:419–424
Sanada Y, Kumoto T, Suehiro H et al (2014) IκB kinase epsilon expression in adipocytes is upregulated by interaction with macrophages. Biosci Biotechnol Biochem 78:1357–1362
Lefterova MI, Zhang Y, Steger DJ et al (2008) PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev 22:2941–2952
Rosen ED, Hsu CH, Wang X et al (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev 16:22–26
Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79:1147–1156
Huq MD, Tsai NP, Lin YP, Higgins L, Wei LN (2007) Vitamin B6 conjugation to nuclear corepressor RIP140 and its role in gene regulation. Nat Chem Biol 3:161–165
Leonardsson G, Steel JH, Christian M et al (2004) Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci U S A 101:8437–8442
Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG (2005) RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol 25:9383–9391
Wu XY, Lu L (2012) Vitamin B6 deficiency, genome instability and cancer. Asian Pac J Cancer Prev 13:5333–5338
Perry C, Yu S, Chen J, Matharu KS, Stover PJ (2007) Effect of vitamin B6 availability on serine hydroxymethyltransferase in MCF-7 cells. Arch Biochem Biophys 462:21–27
Nichols TW Jr, Gaiteri C (2014) Morton’s foot and pyridoxal 5'-phosphate deficiency: genetically linked traits. Med Hypotheses 83:644–648
Nilsson E, Jansson PA, Perfilyev A et al (2014) Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 63:2962–2976
Abbenhardt C, Miller JW, Song X et al (2014) Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J Nutr 144:714–721
Marzio A, Merigliano C, Gatti M, Vernì F (2014) Sugar and chromosome stability: clastogenic effects of sugars in vitamin B6-deficient cells. PLoS Genet 10, e1004199
Verreth W, de Keyzer D, Pelat M et al (2004) Weight-loss-associated induction of peroxisome proliferator-activated receptor-α and peroxisome proliferator-activated receptor-γ correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 110:3259–3269
Petridou A, Tsalouhidou S, Tsalis G, Schulz T, Michna H, Mougios V (2007) Long-term exercise increases the DNA binding activity of peroxisome proliferator-activated receptor γ in rat adipose tissue. Metabolism 56:1029–1036
Floyd ZE, Stephens JM (2012) Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARγ. Biochim Biophys Acta 1822:1090–1095
Shen J, Lai CQ, Mattei J, Ordovas JM, Tucker KL (2010) Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr 91:337–342
Hou CT, Wu YH, Huang PN, Cheng CH, Huang YC (2011) Higher plasma pyridoxal 5'-phosphate is associated with better blood glucose responses in critically ill surgical patients with inadequate vitamin B-6 status. Clin Nutr 30:478–483
Acknowledgements
We acknowledge the technical assistance of I. Alonso, E. Loshuertos and O. Rovira (all from the Department of Diabetes, Endocrinology and Nutrition, IdIBGi, Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partially supported by research grants FIS-PI11/00214, FIS-PI11/1543, FIS-PI12/02631, FIS-PI14/00238 and FIS-PI14/00115 from the Instituto de Salud Carlos III, Spain. CIBEROBN Fisiopatología de la Obesidad y Nutrición is an initiative from the Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional (FEDER) from Spain.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
JMM-N, MJ, MP-O and JMF-R contributed to conception and design, acquisition, analysis and interpretation of data and drafting of the article. FO, GX, WR, EO and RP contributed to acquisition and analysis of data and revised the article critically for important intellectual content. All authors gave their final approval of the version to be published. JMF-R is the guarantor of this work.
Additional information
José María Moreno-Navarrete and Mariona Jove contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 26.6 kb)
ESM Dataset 1
(XLSX 225 kb)
ESM Fig. 1
(PDF 69 kb)
ESM Table 1
(PDF 4 kb)
Rights and permissions
About this article
Cite this article
Moreno-Navarrete, J.M., Jove, M., Ortega, F. et al. Metabolomics uncovers the role of adipose tissue PDXK in adipogenesis and systemic insulin sensitivity. Diabetologia 59, 822–832 (2016). https://doi.org/10.1007/s00125-016-3863-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-016-3863-1