Abstract
Aims/hypothesis
Clinical data regarding circulating leptin levels in patients with non-alcoholic fatty liver disease (NAFLD) are conflicting. The purpose of this meta-analysis was to compare leptin levels between the following groups: patients with biopsy-proven NAFLD vs controls; simple steatosis (SS) patients vs controls; non-alcoholic steatohepatitis (NASH) patients vs controls and NASH patients vs SS patients.
Methods
We performed a systematic search in PubMed, Scopus and the Cochrane Library. We analysed 33 studies, published between 1999 and 2014, including 2,612 individuals (775 controls and 1,837 NAFLD patients).
Results
Higher circulating leptin levels were observed in NAFLD patients vs controls (standardised mean difference [SMD] 0.640; 95% CI 0.422, 0.858), SS patients vs controls (SMD 0.358; 95% CI 0.043, 0.673), NASH patients vs controls (SMD 0.617; 95% CI 0.403, 0.832) and NASH patients vs SS patients (SMD 0.209; 95% CI 0.023, 0.395). These results remained essentially unchanged after excluding studies involving paediatric or adolescent populations and/or individuals undergoing bariatric surgery. There was moderate-to-severe heterogeneity among studies in all comparisons, but no significant publication bias was detected. Meta-regression analysis demonstrated that BMI was inversely associated with leptin SMD and accounted for 26.5% (p = 0.014) and 32.7% (p = 0.021) of the between-study variance in the comparison between NASH patients and controls and NAFLD patients and controls, respectively. However, when bariatric studies were excluded, BMI did not significantly explain the between-study variance.
Conclusions/interpretation
Circulating leptin levels were higher in patients with NAFLD than in controls. Higher levels of circulating leptin were associated with increased severity of NAFLD, and the association remained significant after the exclusion of studies involving paediatric or adolescent populations and morbidly obese individuals subjected to bariatric surgery.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is thought to be the hepatic component of the metabolic or insulin resistance (IR) syndrome; its prevalence is increasing worldwide in parallel with the obesity epidemic and increasing prevalence of type 2 diabetes mellitus [1]. NAFLD ranges from non-alcoholic simple steatosis (SS) to non-alcoholic steatohepatitis (NASH) characterised by steatosis, inflammation and fibrosis. NAFLD is a global public health problem that, apart from its hepatic consequences (liver cirrhosis and/or hepatocellular carcinoma), has systemic complications related to IR syndrome and cardiovascular diseases [2].
Leptin, a 16 kDa protein, is the first adipose-tissue-secreted hormone (adipokine) to be described. Leptin is secreted proportionally to the amount of white adipose mass and is present in the circulation either as the free form or bound to proteins. Its levels in the circulation and adipose tissue depend on the amount of adipose tissue and the status of energy balance; circulating leptin levels reflect primarily body energy stores and secondarily acute changes in energy intake [3]. Leptin plays a crucial role in regulation of energy homeostasis, glucose and lipid metabolism, reproduction and neuroendocrine function [4, 5]. There is also emerging evidence that leptin is involved in cognition, immune function and bone metabolism [6].
IR and adipokines contribute to the pathogenesis of SS and the progression from SS to NASH and NASH-related cirrhosis [1]. However, the specific mechanisms involved remain to be elucidated and the role of leptin in the pathogenesis and progression of NAFLD remains largely unknown. We had previously hypothesised that leptin may have an anti-steatotic effect but that excessive levels may result in hepatic inflammation and fibrosis [7]. Experimental data have recently shown that whereas leptin deficiency may lead to hepatic steatosis (e.g. in ob/ob mice), leptin excess contributes to hepatic inflammation and fibrosis [8]. In this regard, its potential application in patients with NAFLD and NASH will require additional understanding of leptin’s role in lipid handling, hepatic inflammation and fibrosis, along with identification of the potential subsets of patients who might benefit.
Clinical data regarding circulating leptin levels in NAFLD patients are conflicting, with some authors reporting higher levels in NAFLD patients than in controls and others reporting similar levels among groups [9]. Elucidating the role of leptin in NAFLD might have clinical implications, because of the need for non-invasive biomarkers for NASH and NASH-targeting treatment [10, 11].
The aim of this systematic review and meta-analysis was to summarise and compare existing data on circulating leptin levels in biopsy-proven patients with NAFLD (SS and/or NASH) and controls. A deeper insight into the interplay between leptin and NAFLD may lead to the design of new clinical trials aimed at elucidating the potential prognostic and/or therapeutic roles of leptin in NAFLD.
Methods
Literature search
This systematic review was conducted following an a priori established protocol and is reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement [12]. We performed a computerised literature search using the PubMed, Scopus and Cochrane Library electronic databases. The search was not limited by language or publication time. The Medical Subject Heading (MeSH) database was used as a terminological search filter. From the combination of terminological (MeSH terms) and methodological search filters, as proposed elsewhere in detail [13], the following query was formatted: ‘leptin AND (NAFLD OR NASH OR [nonalcoholic fatty liver disease] OR [nonalcoholic steatohepatitis] OR [non-alcoholic fatty liver disease] OR [non-alcoholic steatohepatitis])’ This query was used with minimal differences, according to the requirements of different databases. The full query used in PubMed can be found in electronic supplementary material (ESM) Methods. The literature search was extended by manual search in the ‘related citations’ links of all included articles in PubMed, and the references of all included articles [13].
Inclusion and exclusion criteria
Clinical studies of any design providing comparative circulating leptin levels for NAFLD (or SS or NASH) patients and/or controls were eligible for this systematic review and meta-analysis. Studies were included in the systematic review and meta-analysis, if they met the following criteria: (1) they were original full-text publications; (2) they included biopsy-proven NAFLD patients and (3) they included comparison of circulating leptin levels between NAFLD patients and NAFLD-free controls.
Studies were excluded from the systematic review and meta-analysis on the following grounds: (1) inclusion of patients with other causes of liver disease (e.g. alcoholic fatty liver disease, viral or autoimmune hepatitis) or in whom NAFLD co-existed with other liver disease(s); (2) there was overlap of patients (i.e. the same participants were included in more than one study); (3) additional data were needed, but corresponding authors did not respond; (4) studies were of low methodological quality as defined by a Newcastle–Ottawa scale (NOS) score ≤ 2; (5) studies were interventional with similar comparative groups at baseline (e.g. all groups had SS or NASH at baseline); (6) retrieved articles were reviews, editorials, case reports, letters to the editor, hypotheses, book chapters, studies on animals or cell lines, although letters to the editor providing original data were considered; and (7) unpublished data or abstracts from conferences. Only full-text articles were included, the quality of which is more easily assessed.
In studies that had more than one control group, we included the control group whose characteristics were more similar to the patient group(s), giving priority to BMI and HOMA-IR. For example, if there was one obese and one lean control group and NAFLD patients were obese in a specific study, we included the obese control group in the meta-analysis.
Data extraction
All retrieved articles were saved in an EndNote file (Thompson Reuters, Philadelphia, PA, USA) to facilitate their handling. Two experienced reviewers (SAP and JK), qualified in systematic review and meta-analysis, reviewed independently the title and abstract of all initial search results and excluded studies that did not address the research question, based on our pre-specified inclusion and exclusion criteria. If multiple publications from the same cohort were found, only data from the largest comprehensive study were included. For articles published in a language other than English, a list of required items was requested from the corresponding authors. Subsequently, review of full-text articles and quality assessment was independently carried out by the aforementioned reviewers. A third reviewer (CSM), who was not involved in the previous literature screening and search, was available to adjudicate on any disagreement between the two reviewers.
We extracted the following variables from each study: (1) the study’s general characteristics (reference, name of the first author; year of publication; country where the study was carried out; design); (2) the study’s special characteristics (paediatric/adolescent population; morbidly obese population subjected to bariatric surgery; liver biopsy on the controls; inclusion of patients with NASH-related cirrhosis); (3) patient and/or control characteristics (number, sex, age, BMI, frequency of type 2 diabetes); (4) methods used to evaluate leptin levels and classify NAFLD patients; (5) biochemical measurements (leptin, aspartate aminotransferase [AST], alanine aminotransferase [ALT] and γ-glutamyltranspeptidase [GGT] levels) and (6) evaluation of IR (HOMA-IR).
All corresponding (and/or first authors) of the included studies were contacted by e-mail to request additional data or to request data transformations. Conflicts of data extraction were resolved by consensus after discussion and/or referral back to the corresponding (or first) authors. When corresponding (or first) authors did not respond, transformations were made by standard formulas. If the additional data were considered to be of importance (e.g. many missing values, transformations by formulas not feasible, the performance of liver biopsy, unknown, etc.), and the corresponding author did not respond or was unwilling to provide additional data, the study was excluded from the meta-analysis.
Quality assessment
The quality assessment was performed independently by two reviewers (SAP and JK) using the NOS (Ottawa Hospital Research Institute, Ottawa, ON, Canada). The chance-adjusted inter-rater agreement between the two reviewers was evaluated by the Cohen’s k test. The mentioned third reviewer (CSM) was not involved in the initial quality assessment, but was again available to adjudicate on any disagreement between the two reviewers. The NOS is an instrument that has been developed to assess the quality of observational studies and is frequently used in meta-analyses. It evaluates study validity across three domains: the selection of the study groups; the comparability of the groups and the assessment of the outcome. The quality of the studies is rated on a scale from 0 (very poor) to 9 (high).
Outcomes
The main outcome of the meta-analysis was the standardised mean difference (SMD) of circulating leptin levels between NAFLD patients and NAFLD-free controls. NAFLD patients were further classified as SS or NASH based on Brunt [14] or NAFLD activity score (NAS) [15] histological systems in most studies. Subsequently, we compared leptin levels between the following groups: (1) NAFLD patients vs controls; (2) SS patients vs controls; (3) NASH patients vs controls and (4) NASH patients vs SS patients.
Statistical analysis
The statistical analysis was performed with R3.03 software (R Development Core Team; https://www.r-project.org) and the ‘meta’ package. Given the expected heterogeneity of the outcome, we primarily analysed our data with a random-effects inverse-variance meta-analysis, through the ‘metacont’ routine. We also reported the results from fixed-effects inverse-variance models. We expressed the effect size of the meta-analysis as SMD of leptin levels, and Hedges’ g was used to pool variances for the standardisation. The heterogeneity of the outcome was evaluated using the I 2 statistic. We assessed the existence of any influential study that could be driving the result of the meta-analysis with the ‘metainf’ routine.
We also examined potential publication bias. Publication bias was defined as the tendency of authors and editors to handle studies in which the experimental results achieved statistical significance more favourably when compared with studies in which the results fail to reach significance, which ultimately introduces bias in the literature. We assessed the existence of publication bias with funnel plots and quantified funnel plot asymmetry with Egger’s test for small-study effects, through the ‘metabias’ routine. The Egger’s test performs a linear regression of the SMDs on their standard errors, weighting by the inverse variance of the SMD and testing the null hypothesis of no small-study effect (i.e. no funnel plot asymmetry).
Moreover, we performed univariate, random-effects meta-regression analysis on aggregate-level data in which the SMD was regressed on the following factors: (1) whether or not the diagnosis of controls was biopsy-proven; (2) whether or not patients with NASH-related cirrhosis were included; (3) percentage of male sex; (4) percentage of type 2 diabetes; (5) mean age; (6) mean BMI and (7) mean HOMA-IR of each study’s cohort. Regression coefficients (β) with their p values, and coefficients of determination (R 2, as an index of the heterogeneity accounted for in the model) are reported. All p values were two sided. The α criterion was set at 0.05.
We performed the above-mentioned analysis in the sum of included studies, and then we performed a sensitivity analysis by excluding studies reporting on paediatric/adolescent populations or morbidly obese adults subjected to bariatric surgery, or both. As another sensitivity analysis, we repeated the analyses by using leptin mean difference (MD) instead of SMD.
Results
Literature search
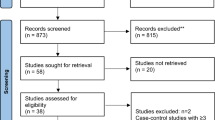
We initially retrieved 435 articles from PubMed, 841 from Scopus and 11 from Cochrane Library (last update 1 January 2015). Sixteen additional articles were added from the ‘related citations’ links and the references of the selected articles. A flowchart, which was prepared according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [16], summarises the identification, screening, eligibility and final selection of the studies included in the meta-analysis (Fig. 1). Forty studies were initially considered eligible for this meta-analysis. We tried to communicate with the corresponding (and/or first) authors of all but one of them (our study), to request additional data. Sixteen of the corresponding (or first) authors provided us with the requested additional/transformed data, whereas 23 authors did not respond (n = 22) or were unwilling to provide additional data (n = 1); seven studies were excluded at the final step because the requested data were considered to be absolutely necessary (ESM Table 1). At the end of the process, 33 studies were included in the meta-analysis.
Characteristics of the included studies
The 33 included studies were published between 1999 and 2014, and reported data from 2,612 individuals (775 controls and 1,837 NAFLD patients) [17–49]. The main characteristics of these studies are presented in Table 1. In 1999, Giannini et al published the first study comparing leptin levels in individuals with biopsy-proven NASH (six men) vs controls (six) [26]. Fifteen studies were carried out in Europe, seven in North America, seven in Asia, two in Australia, one in South America and one in Africa. All studies included in the meta-analysis were cross-sectional. All identified interventional studies had similar comparative groups at baseline (usually NASH) and were excluded. Liver biopsies on controls were performed in five studies. Circulating leptin levels were measured by ELISA in 17 studies, RIA in 13 studies, immunoradiometric assay (IRMA) in two studies and a bead-based immunoassay in one study.
The main demographic and biochemical characteristics per group of each study included in the meta-analysis are presented in ESM Table 2. We reported circulating leptin levels, sex, age, BMI, number of type 2 diabetes patients, liver function tests (AST, ALT and GGT levels) and the HOMA-IR. Data for NAFLD patients were not provided if there was not a control group in the study (i.e. when the study compared leptin levels between SS and NASH patients), since a comparison between NAFLD patients and controls could not be performed. Nine studies included all groups (controls, SS, NASH patients) [18, 28, 30–32, 34, 40, 43, 48]; 11 studies compared leptin levels between NASH patients and controls, without recruiting an SS group [21, 23, 24, 26, 27, 37, 38, 44, 46, 47, 49]; four studies compared leptin levels between NAFLD patients and controls, without providing separate data for SS and NASH [17, 29, 36, 45] and nine studies compared leptin levels between SS and NASH patients, without recruiting a control group [19, 20, 22, 25, 33, 35, 39, 41, 42] (ESM Table 2). Subsequently, comparative data was provided as follows: 24 studies, NAFLD patients (n = 1,231) vs controls (n = 775); nine studies, SS patients (n = 283) vs controls (n = 313); 20 studies, NASH patients (n = 699) vs controls (n = 627) and 18 studies, SS (n = 618) vs NASH patients (n = 702).
Quality of included studies
The quality of included studies according to NOS is presented in Table 1. The chance-adjusted inter-rater agreement between the two reviewers was satisfactory (Cohen’s k = 0.89; p < 0.001). According to NOS results, five studies were scored 3, five studies 4, seven studies 5, 11 studies 6 and five studies 7 (mean ± SD 5.2 ± 1.3). No study was scored ≤ 2, so no study was excluded on the basis of poor NOS score.
Outcomes
Higher circulating leptin levels were observed in the following groups: (1) NAFLD patients vs controls; (2) SS patients vs controls; (3) NASH patients vs controls and (4) NASH vs SS patients (Table 2; Fig. 2). There was moderate-to-severe heterogeneity among studies in all comparisons (I 2 ranged from 59.4% to 77.6%; Fig. 2). No sign of publication bias was detected in any comparison (p > 0.05 for all comparisons; Table 3 and ESM Fig. 1).
In the sensitivity analysis, after excluding paediatric/adolescent studies (n = 3; ESM Fig. 2) or bariatric studies (n = 7; ESM Fig. 3) or both (n = 10; Fig. 3), there were only minimal changes in the comparisons between groups. Heterogeneity among studies remained generally moderate-to-severe (I 2 ranged from 59.7% to 78.5%), apart from the comparison between SS and NASH, which did not remain significant after the exclusion of paediatric studies (I 2 = 21.9%), or both paediatric/adolescent and bariatric studies (I 2 = 9.5%) (Fig. 3 and ESM Figs 2 and 3). No sign of publication bias was detected after excluding paediatric/adolescent or bariatric studies or both (p > 0.05 for all comparisons; Table 3). The results remained essentially unchanged when we used MD instead of SMD in the above-mentioned analyses (ESM Fig. 4).
Forest plots presenting the quantitative synthesis of circulating leptin level, comparing the following groups after excluding studies on paediatric/adolescent populations and morbidly obese adults subjected to bariatric surgery: NAFLD patients vs controls (a); SS patients vs controls (b); NASH patients vs controls (c); NASH patients vs SS patients (d). W, weight
To further investigate the impact of the preselected characteristics on leptin SMD, a random-effects meta-regression analysis was performed. BMI was inversely associated with leptin SMD, when comparing the following groups: (1) NASH patients vs controls (β = −0.027, p = 0.021, R 2 = 32.7%) and (2) NAFLD patients vs controls (β = −0.031, p = 0.014, R 2 = 26.5%). BMI was not associated with leptin SMD in the comparisons between SS and NASH patients (Table 4). Bubble plots demonstrating leptin SMD across different BMI levels in different comparisons between groups are depicted in ESM Fig. 5. When bariatric studies were excluded, BMI was not significantly associated with leptin SMD. The performance of liver biopsy on the controls was inversely associated with leptin SMD when comparing NASH patients with the controls (β = −0.54, p = 0.031, R 2 = 31.5%), whereas it had no effect in the other three comparisons (Table 4). The performance of liver biopsy on controls was not associated with leptin SMD when comparing NASH patients and controls, after excluding bariatric studies. Sex, age, HOMA-IR, type 2 diabetes and inclusion of NASH-related cirrhosis within the NASH group were not associated with leptin SMD (ESM Table 3).
Discussion
This meta-analysis demonstrated that high levels of leptin in the circulation were associated with the severity of NAFLD; controls had lower leptin levels than SS, NASH or NAFLD patients, and SS patients had lower leptin levels than NASH patients. The sensitivity analyses did not essentially change these findings.
The meta-regression analysis revealed that BMI was inversely associated with leptin SMD when comparing patients with NAFLD (or NASH) and controls, and that this association was no longer significant after the exclusion of morbidly obese patients. This may be partly attributed to differences in BMI between non-morbidly obese and morbidly obese adults subjected to bariatric surgery (Table 4). In any case, the magnitude of the association (β), although statistically significant, was low (−0.031 and −0.027, respectively), thereby possibly being of low clinical significance. The performance of liver biopsy in controls was also inversely associated with leptin SMD when comparing NASH patients and controls, and this association also lost its significance after the exclusion of morbidly obese patients. This seems reasonable given that, except for morbidly obese patients (and controls) subjected to bariatric surgery, liver biopsy in controls was performed only in two studies [29, 34]; thus, when the majority of studies were excluded, the remaining two did not significantly add to the between-study heterogeneity. Leptin SMD was not associated with sex, age, HOMA-IR, type 2 diabetes or the inclusion of NASH-related cirrhosis within NASH group.
Experimental data have shown that leptin deficiency may lead to hepatic steatosis (e.g. in ob/ob mice), which can be reversed by leptin replacement [9]. On the other hand, excess leptin may contribute to hepatic inflammation and fibrosis [8, 9]. However, this potential dual role of leptin cannot be proven by this meta-analysis of cross-sectional studies and it remains to be elucidated in large prospective cohort studies with paired biopsies.
Although liver biopsy is considered the gold standard for the assessment of liver fat, inflammation and fibrosis, there is a need for less invasive diagnostic techniques to facilitate diagnosis and avoid complications related to liver biopsy [10]. This meta-analysis cannot answer the question of whether circulating leptin could serve as a surrogate non-invasive marker for NASH, because this answer requires studies of different design. However, this meta-analysis could be a trigger for the design of clinical trials investigating whether leptin, alone or in combination with other variables, could serve as non-invasive index. There are limited data on whether leptin could be used as a non-invasive biomarker for NAFLD, and more research in this area is needed.
The results of this meta-analysis do not warrant the design of trials with recombinant leptin in NASH patients with normoleptinaemia or hyperleptinaemia. NASH patients have high leptin levels and, based on experimental studies, leptin administration might have an adverse effect on liver fibrosis, thereby possibly worsening the prognosis of the disease. In line, to the best of our knowledge, there is no ongoing study evaluating the effect of recombinant leptin administration in NASH patients with normoleptinaemia or hyperleptinaemia. On the contrary, NAFLD patients with hypoleptinaemia, being the minority of NAFLD patients, might possibly benefit from leptin replacement. The first evidence comes from uncontrolled studies with lipodystrophic patients with NAFLD [9]; leptin seems to be a highly effective treatment for NASH in hypoleptinaemic lipodystrophic patients, but this remains to be fully proven by future long-term and well-controlled studies.
This meta-analysis has certain limitations. First, all included studies were observational (cross-sectional), therefore a cause–effect relationship between circulating leptin and NAFLD severity cannot be shown. There are limited data from longitudinal cohort studies providing evidence on leptin changes when NAFLD progresses or regresses over time [9]. These studies have been inconclusive and are primarily limited by the high dropout rates, because of the need for repeat liver biopsy. Second, the between-study heterogeneity limits the reliability of the results, which should be cautiously interpreted, especially in regard to BMI differences between groups being compared. Third, we used BMI and not waist circumference as a measure of adiposity, mainly because waist circumference was not recorded in many of the included studies. However, since leptin reflects total body fat, BMI can serve as a sufficient measure of adiposity. Fourth, by excluding unpublished studies or abstracts from conferences, a publication bias might exist. Excluding such studies, however, is essential to avoid introduction of low-quality data, since the quality of unpublished data and abstracts cannot be fully reviewed [50]. Fifth, there is no well-validated quality-assessment tool for cross-sectional studies. We used the most commonly utilised scale for observational studies (NOS), aiming to exclude studies with very poor quality. Sixth, some studies included patients with NASH-related cirrhosis in the NASH group; we did not exclude these studies, because we considered that the selection bias might outweigh the effect that a minority of patients with NASH-related cirrhosis on circulating leptin levels might have, if any. Furthermore, most studies included only a small sample of cirrhotic patients (Table 1). Patients with NASH-related cirrhosis were not examined separately in this meta-analysis, since none of the studies examined these patients as a separate group. Leptin levels have been elevated in the majority of studies examining patients with cirrhosis of other or mixed (including NASH-related) aetiology [9]. Seventh, the lack of liver biopsy in controls was not an exclusion criterion because a selection bias might have been committed; since liver biopsy in controls meets ethical considerations, controls were recruited on the basis of normal liver ultrasound and serum aminotransferases levels in most studies. On the other hand, another type of selection bias may also be committed in most studies in which controls were subjected to liver biopsy, because they were selected populations (e.g. morbidly obese patients subjected to bariatric surgery or patients subjected to laparoscopic cholecystectomy or hepatectomy for benign tumours) (Table 1). Eighth, the quality of the included case–control studies might be also limited due to the fact that only three studies explicitly mentioned that the controls were drawn from the same pool as the cases (Table 1). Ninth, we did not examine leptin levels separately for steatosis grade or inflammation or fibrosis stage, since only a minority of studies provided data for specific histological lesions; this was further limited by the studies’ different histological definitions and group division. Finally, none of the included studies reported a risk ratio for different levels of leptin, so we were not able to evaluate a potential non-linear association of leptin.
In conclusion, this meta-analysis demonstrated that a high level of circulating leptin was associated with the severity of NAFLD. These results are consistent with the notion of an anti-steatotic action of leptin during the initial stages of NAFLD and a pro-inflammatory and fibrotic action during disease progression. This speculation, which has been shown in experimental models of NAFLD, remains to be elucidated in humans by prospective cohort studies with paired biopsies. This meta-analysis also warrants further studies examining the role of leptin, alone or in combination with other variables, as a non-invasive biomarker for NAFLD.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- GGT:
-
γ-Glutamyltranspeptidase
- IR:
-
Insulin resistance
- IRMA:
-
Immunoradiometric assay
- MD:
-
Mean difference
- NAFLD:
-
Non-alcoholic fatty liver disease
- NAS:
-
NAFLD activity score
- NASH:
-
Non-alcoholic steatohepatitis
- NOS:
-
Newcastle–Ottawa scale
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- SMD:
-
Standardised MD
- SS:
-
Simple steatosis
References
Polyzos SA, Kountouras J, Zavos C (2009) Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med 72:299–314
Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34:274–285
Kelesidis T, Kelesidis I, Chou S, Mantzoros CS (2010) Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152:93–100
Moon HS, Dalamaga M, Kim SY et al (2013) Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev 34:377–412
Mantzoros CS, Magkos F, Brinkoetter M et al (2011) Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 301:E567–E584
Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS (2013) Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab 18:29–42
Polyzos SA, Kountouras J, Zavos C, Deretzi G (2011) The potential adverse role of leptin resistance in nonalcoholic fatty liver disease: a hypothesis based on critical review of literature. J Clin Gastroenterol 45:50–54
Imajo K, Fujita K, Yoneda M et al (2012) Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab 16:44–54
Polyzos SA, Kountouras J, Mantzoros CS (2015) Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism 64:60–78
Polyzos SA, Mantzoros CS (2014) Necessity for timely noninvasive diagnosis of nonalcoholic fatty liver disease. Metabolism 63:161–167
Polyzos SA, Mantzoros CS (2015) Leptin in health and disease: facts and expectations at its twentieth anniversary. Metabolism 64:5–12
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J (2011) Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism 60:313–326
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474
Kleiner DE, Brunt EM, Van NM et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD (2004) Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol 41:943–949
Argentou M, Tiniakos DG, Karanikolas M et al (2009) Adipokine serum levels are related to liver histology in severely obese patients undergoing bariatric surgery. Obes Surg 19:1313–1323
Canbakan B, Tahan V, Balci H et al (2008) Leptin in nonalcoholic fatty liver disease. Ann Hepatol 7:249–254
Cayon A, Crespo J, Mayorga M, Guerra A, Pons-Romero F (2006) Increased expression of Ob-Rb and its relationship with the overexpression of TGF-β1 and the stage of fibrosis in patients with nonalcoholic steatohepatitis. Liver Int 26:1065–1071
Chalasani N, Crabb DW, Cummings OW et al (2003) Does leptin play a role in the pathogenesis of human nonalcoholic steatohepatitis? Am J Gastroenterol 98:2771–2776
Charlton M, Angulo P, Chalasani N et al (2008) Low circulating levels of dehydroepiandrosterone in histologically advanced nonalcoholic fatty liver disease. Hepatology 47:484–492
Chitturi S, Farrell G, Frost L et al (2002) Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology 36:403–409
Dasarathy S, Yang Y, McCullough AJ, Marczewski S, Bennett C, Kalhan SC (2011) Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol 23:382–388
Fitzpatrick E, Dew TK, Quaglia A, Sherwood RA, Mitry RR, Dhawan A (2012) Analysis of adipokine concentrations in paediatric non-alcoholic fatty liver disease. Pediatr Obes 7:471–479
Giannini E, Botta F, Cataldi A et al (1999) Leptin levels in nonalcoholic steatohepatitis and chronic hepatitis C. Hepatogastroenterology 46:2422–2425
Gonciarz M, Bielanski W, Partyka R et al (2013) Plasma insulin, leptin, adiponectin, resistin, ghrelin, and melatonin in nonalcoholic steatohepatitis patients treated with melatonin. J Pineal Res 54:154–161
Haukeland JW, Dahl TB, Yndestad A et al (2012) Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol 166:503–510
Huang XD, Fan Y, Zhang H et al (2008) Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. World J Gastroenterol 14:2888–2893
Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J (2004) Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 40:46–54
Kashyap SR, Diab DL, Baker AR et al (2009) Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring) 17:1696–1701
Koehler E, Swain J, Sanderson S, Krishnan A, Watt K, Charlton M (2012) Growth hormone, dehydroepiandrosterone and adiponectin levels in non-alcoholic steatohepatitis: an endocrine signature for advanced fibrosis in obese patients. Liver Int 32:279–286
Le D, Marks D, Lyle E et al (2007) Serum leptin levels, hepatic leptin receptor transcription, and clinical predictors of non-alcoholic steatohepatitis in obese bariatric surgery patients. Surg Endosc 21:1593–1599
Lemoine M, Ratziu V, Kim M et al (2009) Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int 29:1431–1438
Machado MV, Coutinho J, Carepa F, Costa A, Proenca H, Cortez-Pinto H (2012) How adiponectin, leptin, and ghrelin orchestrate together and correlate with the severity of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 24:1166–1172
Manco M, Giordano U, Turchetta A et al (2009) Insulin resistance and exercise capacity in male children and adolescents with non-alcoholic fatty liver disease. Acta Diabetol 46:97–104
Munoz LE, Cordero P, Torres L, Sauceda AY, Flores JP, Segura JJ (2009) Adipokines in a group of Mexican patients with nonalcoholic steatohepatitis. Ann Hepatol 8:123–128
Musso G, Gambino R, Durazzo M et al (2005) Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology 42:1175–1183
Nobili V, Manco M, Ciampalini P et al (2006) Leptin, free leptin index, insulin resistance and liver fibrosis in children with non-alcoholic fatty liver disease. Eur J Endocrinol 155:735–743
Pagano C, Soardo G, Esposito W et al (2005) Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol 152:113–118
Petta S, Amato MC, Di Marco V et al (2012) Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 35:238–247
Pirvulescu I, Gheorghe L, Csiki I et al (2012) Noninvasive clinical model for the diagnosis of nonalcoholic steatohepatitis in overweight and morbidly obese patients undergoing bariatric surgery. Chirurgia (Bucur) 107:772–779
Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS (2014) Irisin in patients with nonalcoholic fatty liver disease. Metabolism 63:207–217
Singh DK, Sakhuja P, Rastogi A, Singh A, Gondal R, Sarin SK (2013) Serum leptin levels correlate with body mass index but not with histologic disease severity in Indian patients with non-alcoholic steatohepatitis: a pilot study. Indian J Med Res 137:986–987
Swellam M, Hamdy N (2012) Association of nonalcoholic fatty liver disease with a single nucleotide polymorphism on the gene encoding leptin receptor. IUBMB Life 64:180–186
Tungtrongchitr R, Treeprasertsuk S, Ei NN, Thepouyporn A, Phonrat B, Huntrup A (2006) Serum leptin concentrations in chronic hepatitis. J Med Assoc Thail 89:490–499
Uygun A, Kadayifci A, Yesilova Z et al (2000) Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 95:3584–3589
Wong VW, Hui AY, Tsang SW et al (2006) Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 4:1154–1161
Yalniz M, Bahcecioglu IH, Ataseven H et al (2006) Serum adipokine and ghrelin levels in nonalcoholic steatohepatitis. Mediat Inflamm 2006:34295
Egger M, Juni P, Bartlett C, Holenstein F, Sterne J (2003) How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 7:1–76
Acknowledgements
We are sincerely grateful to P. Angulo (Mayo Clinic College of Medicine, USA), M. Argentou (University of Patras, Greece), N. Chalasani (Indiana University School of Medicine, USA), S. Chitturi (University of Sydney, New Zealand), S. Dasarathy (Cleveland Clinic, USA), E. Giannini (University of Genova, Italy), M. Gonciarz (St Barbara’s Main District Hospital, Poland), J. W. Haukeland (University of Oslo, Norway), S. R. Kashyap (Cleveland Clinic, USA), M. V. Machado (Hospital of Santa Maria, Portugal), R. W. O’Rourke and D. Marks (Oregon Health and Science University, USA), S. Petta (University of Palermo, Italy), I. Pirvulescu (Fundeni Clinical Institute, Romania), L. Serfaty and M. Lemoine (University of Pierre and Marie Curie, France), V. W. Wong and H. L. Chan (Chinese University of Hong Kong, China) and M. Yalniz (Firat University, Turkey) for their willingness to provide additional data on their studies, regarded as being invaluable to this work.
Duality of interest
CSM has served as a consultant for Astra Zeneca and has received research support through his Institution from Amgen and a lecture fee from Aegerion. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
SAP, JK and CSM were responsible for the study concept and design. SAP, DDR and MFV acquired data. Data were interpreted by SAP and KNA and statistical analysis was carried out by KNA. The manuscript was drafted by SAP and critically revised for important intellectual content by all authors. All authors approved the final version of the manuscript to be published. SAP is the guarantor of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 49 kb)
ESM Fig. 1
(PDF 94 kb)
ESM Fig. 2
(PDF 1536 kb)
ESM Fig. 3
(PDF 1320 kb)
ESM Fig. 4
(PDF 5555 kb)
ESM Fig. 5
(PDF 137 kb)
ESM Table 1
(PDF 73 kb)
ESM Table 2
(PDF 137 kb)
ESM Table 3
(PDF 84 kb)
Rights and permissions
About this article
Cite this article
Polyzos, S.A., Aronis, K.N., Kountouras, J. et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 59, 30–43 (2016). https://doi.org/10.1007/s00125-015-3769-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-015-3769-3