Abstract
Aims/hypothesis
Protein tyrosine phosphatase non-receptor 22 (PTPN22) plays a central role in T cell, B cell and innate immune cell signalling. A genetic variation in Ptpn22 is considered a major risk factor for the development of type 1 diabetes and has been the subject of extensive study. While several reports have addressed how Ptpn22 might predispose to autoimmunity, its involvement in other immune-mediated diseases, such as allograft rejection, has not been explored.
Methods
To address a possible function for Ptpn22 in allograft rejection, we used a mouse model of pancreatic islet transplantation. We performed transplant tolerance experiments and determined how PTPN22 shapes tolerance induction and maintenance.
Results
Ptpn22 −/− recipient mice generate higher numbers of alloreactive T cells after allogeneic pancreatic islet transplantation compared with wild-type (WT) mice, but reject grafts with similar kinetics. This is not only due to their well-documented increase in forkhead box protein P3 (FOXP3)+ T regulatory (Treg) cells but also to the expansion of T regulatory type 1 (Tr1) cells caused by the lack of PTPN22. In addition, a tolerogenic treatment known to induce transplant tolerance in WT mice via Tr1 cell generation is more effective in Ptpn22 −/− mice as a consequence of boosting both Tr1 and FOXP3+ Treg cells.
Conclusions/interpretation
A lack of PTPN22 strengthens transplant tolerance to pancreatic islets by expanding both FOXP3+ Treg and Tr1 cells. These data suggest that targeting PTPN22 could serve to boost transplant tolerance.
Similar content being viewed by others
Introduction
The autoimmune predisposing gene Ptpn22 is involved in several aspects of immune regulation [1–4]. Ptpn22 encodes a protein tyrosine phosphatase involved in T cell receptor (TCR) [5–8], B cell receptor [9–11] and innate immune signalling [12]. A strong link between the R620W genetic variant of Ptpn22 and the development of autoimmunity was made a decade ago [13–15]. Since then, healthy donors and patients, carriers of PTPN22*W, and genetically modified mice have been studied, with contradictory results reported. Some studies have suggested that PTPN22*W is a loss of function variant associated with increased T cell signalling [16], while others have suggested the opposite, i.e. that the polymorphism increases phosphatase activity, resulting in reduced T cell activation [17, 18].
Of the several mouse models that have served to address the role of protein tyrosine phosphatase non-receptor 22 (PTPN22), two Ptpn22 knockout (Ptpn22 −/−) mouse lines were reported that, despite having increased numbers of memory T cells, spontaneous germinal centres and elevated serum immunoglobulin concentrations compared with wild-type (WT) mice, showed no signs of autoimmunity [7, 19]. However, when crossed with another tyrosine phosphatase mutant mouse model, the CD45 E613R, Ptpn22 −/− mice developed lupus-like disease [20]. Ptpn22 −/− mice contained an increased proportion and greater absolute number of forkhead box protein P3 (FOXP3)+ T regulatory (Treg) cells [19, 21], and Treg cell transfer from Ptpn22 −/− mice controlled experimental colitis [19]. Ptpn22 −/− mice were resistant to experimental autoimmune encephalomyelitis [21] but more susceptible to dextran sulphate induced colitis [12, 22] and experimental arthritis [23]. NOD mice with knocked down expression of PTPN22 by RNA interference [24] or, in contrast, with transgenic overexpression of PTPN22 [25] were protected from type 1 diabetes mellitus. These results indicate that PTPN22 can promote or hinder tolerance depending on the experimental setting, which possibly depends on the type of effector cells that promote inflammation [3].
Various types of peripherally derived T regulatory (pTreg) cells have been described. The best characterised subsets are the FOXP3+ pTreg and T regulatory type 1 (Tr1) cells [26, 27]. Tr1 cells are characterised by their high expression of IL-10 and lack of constitutively expressed FOXP3, and can be identified by surface expression of lymphocyte activation gene 3 (LAG-3) and CD49b [28, 29]. It is not known whether PTPN22 is involved in the induction, maintenance and function of Tr1 cells. Given that PTPN22 controls FOXP3+ Treg cell peripheral induction and level of IL-10 production [19], we investigated the role of PTPN22 in allograft rejection and Tr1 cell mediated transplant tolerance using a mouse model of pancreatic islet transplantation.
Methods
Mice
BALB/c, C3H and C57BL/6 mice were purchased from Charles River (Calco, Italy). Homozygous Ptpn22-deficient mice were previously described [7]. WT and Ptpn22 −/− littermates were used throughout the study. All animals were housed under specific pathogen free conditions in compliance with guidelines of the San Raffaele Institutional Animal Care and Use Committee (IACUC number: 479).
Islet transplantation and treatments
Pancreatic islets from BALB/c or C3H donors were transplanted under the kidney capsule of chemically (streptozotocin) induced diabetic recipient mice, as previously described [30]. Mice transplanted with BALB/c islets and receiving no treatment are defined as Tx. Transplanted mice were treated s.c. with 200 mg/kg granulocyte-colony stimulating factor (G-CSF; Myelostim, rHuG-CSF, Italfarmaco, Milan, Italy) and orally with 1 mg/kg rapamycin (RAPA; Rapamune; Wyeth Europe, Taplow, UK) daily for 30 consecutive days, as previously described [30] and boosted 60 days after i.p. transplantation with 30 × 106 splenocytes of the donor. Mice normoglycaemic for ≥15 days after the boost were considered tolerant (TxT). Eight 12-week-old unmanipulated (UM) mice were used as (steady-state) controls. Anti-LAG-3 (clone C9B7W; i.v. 200 μg/dose, two doses per week) and anti-CD25 (clone PC61; i.v. 500 μg/dose, five daily doses) monoclonal antibody (mAb) treatments were given. P60 (a 15-mer synthetic peptide that can bind to and block FOXP3, i.p. 100 μg/dose daily, up to 10 doses) was given, as previously described [31]. For splenectomy, animals were anesthetised and the spleen artery and vein were clamped and cauterised prior to excision.
Blood glucose monitoring
Blood glucose was monitored every other day with a monitoring system (OneTouch Ultra; LifeScan, Milpitas, CA, USA). Diabetes was defined as two consecutive blood glucose values above 13.89 mmol/l.
Flow cytometry staining
Cell surface staining was performed with anti-mouse CD3, CD4, CD25, CD8, LAG-3, CD49b lymphocyte function-associated antigen 1 (LFA-1), CD62L, and CD44 mAbs (all from BD Pharmingen, San Diego, CA, USA) following a 2.4G2 (Fc) blocking step. FOXP3, IL-10, IFN-γ, IL-4, IL-2, TNF-α and cytotoxic T-lymphocyte protein 4 (CTLA-4) were tested after fixation with the FOXP3 staining kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Cells were processed using a FACSCanto (BD Bioscience) and analysed with FlowJo (Tree Star, USA) software.
Cell isolation and adoptive transfer experiments
CD4+LAG-3+ or CD4+LAG-3− T cells (2.5 × 105/recipient) from the spleens of TxT or UM mice were subjected to FACS or bead-purification (CD4-negative isolation followed by LAG-3-phycoerythrin (PE) staining and anti-PE microbead isolation, Miltenyi Biotec, San Diego, CA, USA) prior to adoptive transfer into recipients 1 day before transplantation. In ELIspot experiments, T cells were positively purified with CD90.2 microbeads (Miltenyi Biotec), whereas T cell-depleted splenocytes (TDS) were prepared by depletion with CD90.2 microbeads. TDS from C57BL/6, C3H or BALB/c mice were irradiated at 3,000 rads before co-culture.
ELIspot immunoassay
ELIspot for IFN-γ (BD Pharmingen) was performed as previously described [32]. Briefly, 96-well millititre HA plates (Millipore, Bedford, MA, USA) were coated with 5 μg/ml IFN-γ capture antibody. After a 10% (vol./vol.) FBS/RPMI blocking step, CD90.2-purified T cells from the spleens of tolerant mice were plated at 2 × 105/well and cultured for 3 days in the presence of an equal number of C57BL/6, BALB/c or C3H (third donor control) irradiated TDS. Plates scanned and spots representing cytokine-producing cells were counted using an Eli.Expert ELIspot Reader and analysed using Eli.Analyze software version 5.1 (A.EL.VIS, Cologne, Germany).
Statistical analysis
Comparisons between groups were performed using the unpaired, two-tailed Student’s t test or the Mann–Whitney nonparametric U test, whereas the Kruskal–Wallis test was used for multiple comparisons. Islet allograft survival was determined by Kaplan–Meier survival curves and compared using the logrank test. Prism software (GraphPad, USA) was used for all analyses. Statistical significance is indicated by *p < 0.05, **p < 0.01 and ***p < 0.001.
Results
Blockade of Treg cell function accelerates islet allograft rejection in WT but less so in Ptpn22 −/− mice
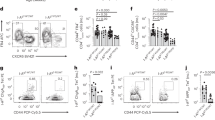
In the steady state, Ptpn22 −/− mice contain an increased frequency and number of CD4+FOXP3+ Treg cells compared with WT mice (Fig. 1a–c), as previously described [19, 21]. The increase in Treg cells was still present in Ptpn22 −/− mice 14 days after transplantation of allogeneic islets (Tx; Fig. 1a–c). Treg cells had already increased in frequency and number in the thymus of Ptpn22 −/− mice, in line with a previous report (see electronic supplementary material [ESM] Fig. 1) [21]. CD4 single positive, CD4/CD8 double negative and double positive, and positive selection stages (defined by CD69 and TCRβ) were moderately but significantly altered by the absence of PTPN22 (ESM Fig. 1).
Ptpn22 −/− transplanted mice show increased expansion of FOXP3+ Treg and alloreactive T cells. (a–c) The frequency and number of CD4+FOXP3+ Treg cells in the spleens of 8–12-week-old WT and Ptpn22 −/− (KO) mice in the steady state and 14 days after transplantation (Tx). (a) Representative plots show the percentages of FOXP3+ cells in individual mice. (b, c) Graphs show the results from spleens analysed from multiple mice (n = 4/group) ± SEM. WT, open circles; KO, open squares; WT Tx, black circles; KO Tx, black squares. (d, e) Number of alloreactive T cells in WT Tx and KO Tx mice 14 days after transplantation, measured by ELIspot. Representative wells show the number of IFN-γ+ T cells activated with TDS from C57BL/6, BALB/c or C3H mice. UM WT and KO mice were used as controls. (f) Graft rejection was monitored in WT Tx, black line with closed circles (n = 10); KO Tx, grey line with closed squares (n = 10); WT Tx + P60, black dashed line with open circles (n = 4); and KO Tx + P60 mice, grey dashed line with open squares (n = 5). *p < 0.05, ***p < 0.001; NS, not statistically significant
An increased number of splenocytes and increased frequency of memory T cells were detected in Ptpn22 −/− mice in the steady state (ESM Fig. 2 and data not shown), as previously described [7]. Frequencies of memory CD4+ T cells (i.e. CD44hiCD62Llo) and central memory (i.e. CD44hiCD62L+), but not of effector memory (i.e. CD44hiCD62Llo), CD8+ T cells remained higher in Ptpn22 −/− mice 14 days after transplantation (ESM Fig. 2). The frequency of naive (i.e. CD44intCD62L+) CD4+ and CD8+ T cells was lower in Ptpn22 −/− mice in the steady state (ESM Fig. 2). However, the ratio of naive to memory T cells was not maintained at WT levels in the absence of PTPN22 (ESM Fig. 2). The number of IFN-γ-producing BALB/c-specific T cells was significantly increased in Ptpn22 −/− Tx mice (Fig. 1d, e), suggesting that a lack of PTPN22 favours the generation of alloreactive T cells.
The kinetics of pancreatic islet graft rejection between Ptpn22 −/− and WT mice was similar (BALB/c → WT vs BALB/c → Ptpn22 −/−; Fig. 1f). Given that the increased number of allogeneic T cells in Ptpn22 −/− mice might be compensated for by the enlarged number of Treg cells, P60 (a peptide that specifically blocks FOXP3) was administered [33]. WT P60-treated Tx mice rejected grafts much faster than untreated Tx mice; however, P60 treatment did not accelerate graft rejection to similar levels in Ptpn22 −/− mice (Fig. 1f and Table 1). These results show that FOXP3 blockade is less effective in accelerating graft rejection in Ptpn22 −/− mice, thus suggesting that additional regulatory mechanisms might be active in the absence of PTPN22.
Lack of PTPN22 increases Tr1 cells that control allograft rejection
We focused our subsequent analyses on Tr1 cells. LAG-3+ and IL-10+ (FOXP3−) splenic Tr1 cells were substantially increased in frequency and number in Ptpn22 −/− mice in both the steady state and 14 days after transplantation (Tx) (Fig. 2a–d). The results are similar when frequencies of double positive LAG-3+CD49b+ cells are compared (ESM Fig. 3). To address whether Tr1 cells are involved in allograft rejection in Ptpn22 −/− mice, LAG-3 was blocked by mAb administration [34]. Anti-LAG-3 mAb treatment significantly accelerated islet allograft rejection in Ptpn22 −/− Tx mice, and had a similar effect in WT Tx mice (Fig. 2e, f and Table 2). Combined anti-LAG-3 mAb and FOXP3 blockade also accelerated graft rejection; however, this did not occur faster than with anti-LAG-3 mAb alone (Fig. 2e, f and Table 2). Since LAG-3 can be also expressed by a subset of pTreg cells, its expression was examined in conjunction with CD25. The majority of LAG-3+ CD4+ T cells are CD25−, showing that anti-LAG-3 mAb treatment primarily blocked Tr1 cells (ESM Fig. 4). Thus, in addition to Treg cells, Tr1 cells are expanded in Ptpn22 −/− mice and play a central role in controlling allograft rejection.
Ptpn22 −/− mice have an increased frequency and number of Tr1 cells that control allograft rejection. (a–c) Frequency and number of CD4+LAG-3+ Tr1 cells in the spleens of 8–12-week-old WT and Ptpn22 −/− (KO) mice at steady state and 14 days after transplantation (Tx). (a) Representative plots show the percentages of Tr1 cells in individual mice. (b, c) Graphs show the results from the spleens from multiple mice (n = 4/group) ± SEM. WT, open circles; KO, open squares; WT Tx, black circles; KO Tx, black squares. (d) Representative plots show the frequency of IL-10-producing cells in WT, KO, WT Tx and KO Tx mice. (e) Graft rejection in WT Tx, black line with closed circles (n = 10); WT Tx + anti-LAG-3, grey line with closed circle (n = 3); and WT Tx + anti-LAG-3 + P60, black line with open circle (n = 4). (f) Graft rejection in KO Tx, black line with closed squares (n = 10); KO Tx + anti-LAG-3, grey line with closed square (n = 3) and KO Tx + anti-LAG-3 + P60, black line with open square (n = 4). *p < 0.05
G-CSF/RAPA treatment in Ptpn22 −/− mice induces transplant tolerance that is maintained after Treg depletion or blockade
We previously described an efficient immune-modulatory protocol that combines G-CSF/RAPA and leads to pancreatic islet transplant tolerance in WT mice via Tr1 cells [30]. Thus, this experimental model was used to evaluate the role of PTPN22 in Tr1 cell mediated transplant tolerance. G-CSF/RAPA treatment led to stable long-term transplant tolerance in 100% of Tx mice (Fig. 3a). A higher frequency of memory phenotype CD4+ and CD8+ T cells was found in the spleen but not in kidney draining lymph nodes (dLNs) or the grafts of Ptpn22 −/−TxT mice (Fig. 3b–f). Given that memory T cell numbers were increased in the spleens of Ptpn22 −/− mice in the steady state (ESM Fig. 2), the fold change was calculated. Lack of PTPN22 did not affect the expansion of splenic memory CD4+ and central memory CD8+ T cells (shown in Fig. 3c, e, f). However, effector memory CD8+ splenocytes showed a greater expansion in Ptpn22 −/− TxT mice (Fig. 3e).
G-CSF/RAPA induces robust transplant tolerance in Ptpn22 −/− mice. (a) WT and Ptpn22 −/− (KO) mice underwent transplantation with BALB/c islets. Control mice were untreated (WT Tx, black line with closed circle, n = 6; KO Tx, grey line, n = 6) or treated with G-CSF/RAPA (WT TxT, black line with open circle, n = 9; KO TxT, grey dashed line, n = 9). Sixty days after transplantation, 30 × 106 splenocytes isolated from BALB/c mice were injected into TxT mice (boost). Overall graft survival is shown. (b, c) Frequency of CD44hiCD62Llo CD4+ T cells in the spleens, kidney dLNs and grafts of TxT WT and KO mice 100 days after transplantation. (b) Representative plots show the percentage of memory CD4+ splenocytes in individual mice. (c) Graphs show the results from multiple mice (n = 3–6/group) ± SEM. WT TxT, open circles; KO TxT, open squares. (d–f) Frequency of CD44hiCD62Llo and CD44hiCD62L+ CD8+ T cells in the spleens, kidney dLNs and grafts of WT and KO TxT mice 100 days after transplantation. (d) Representative plots are shown. Numbers in parentheses indicate the average fold change compared with UM 8–12-week-old mice. *p < 0.05, **p < 0.01; NS, not statistically significant
Treg cells were found at a higher frequency in the spleens and kidney dLNs but not in the grafts of Ptpn22 −/− TxT mice (Fig. 4a–c). Splenic Treg cells expansion was similar in WT and Ptpn22 −/− TxT mice (on average, by 1.5 times compared with the steady state; Fig. 4b, c). To address whether G-CSF/RAPA treatment promotes long-term transplant tolerance through Treg cells, Treg cells were depleted with anti-CD25 mAb or blocked with P60 [31]. Anti-CD25 mAb reduced the frequency of circulating CD4+FOXP3+ Treg cells by approximately 60% in WT TxT mice 10 days after treatment (ESM Fig. 5), in line with previous reports [35]. None of the treatments modified G-CSF/RAPA-induced transplant tolerance in Ptpn22 −/− mice (Fig. 4e, f), similar to our previous data in WT mice [30]. Thus, G-CSF/RAPA treatment induces transplant tolerance in Ptpn22 −/− mice, which is maintained upon Treg cell depletion or blockade.
G-CSF/RAPA induces FOXP3+ Treg cells, which are dispensable for tolerance maintenance in Ptpn22 −/− mice. (a–c) Frequency of FOXP3+CD4+ T cells in the spleens of G-CSF/RAPA-treated WT and Ptpn22 −/− (KO) TxT mice 100 days after transplantation. (a) Representative plots show the percentages of FOXP3+ Treg cells in individual mice. (b, c) Graphs show results from the spleens, kidney dLNs and grafts of multiple mice (n = 3–6/group) ± SEM. WT TxT, open circles; KO TxT, open squares. Numbers in parenthesis indicate the average fold change compared with 8–12-week-old UM mice. (d) WT and KO TxT mice were depleted of Treg cells with anti-CD25 mAb starting >80 days after transplantation. Overall graft survival is shown in WT TxT, black line with closed circle (n = 4); WT Tx + anti-CD25, black line with open circle (n = 8); KO TxT, grey line with closed square (n = 4); KO TxT + anti-CD25 (grey dashed line with open square, n = 4). (e) FOXP3 was blocked in WT and KO TxT mice by P60 peptide treatment. Experimental groups are the same as in (d), WT TxT + P60 (n = 3); KO TxT + P60 (n = 4). *p < 0.05, ***p < 0.001; NS, not statistically significant
G-CSF/RAPA treatment in Ptpn22 −/− mice induces Tr1 cells that transfer transplant tolerance in an antigen-specific manner
Tr1 cell frequency was evaluated in the spleens, kidney dLNs and grafts of TxT mice. LAG-3+, LAG-3+CD49b+ and IL-10-producing CD4+ T cells were found at higher frequencies in the spleens of Ptpn22 −/− TxT mice, but were present at similar frequencies in kidney dLNs and were almost undetectable in the graft of both WT and Ptpn22 −/− TxT mice (Fig. 5a–c, ESM Fig. 3 and data not shown). A 3.5-fold Tr1 cell expansion, which is much greater than the amount of Treg cell expansion, was observed in the spleen of TxT mice. However, a lack of PTPN22 had no effect on the expansion or induction of Tr1 cells (Fig. 5b).
G-CSF/RAPA induces splenic Tr1 cells in Ptpn22 −/− mice that transfer transplant tolerance. (a–c) Frequency of CD4+LAG-3+ and CD4+IL-10+ (FOXP3−) Tr1 cells in the spleens of WT and Ptpn22 −/− (KO) TxT mice 100 days after transplantation. (a) Representative plots show the percentages of LAG-3+ Tr1 cells in individual mice. (b, c) Graphs show results from the spleens, kidney dLNs and grafts of multiple mice (n = 3–6/group) ± SEM. WT TxT, open circles; KO TxT, open squares. Numbers in parenthesis indicate the average fold change compared with 8–12-week-old UM mice. (d) CD4+LAG-3+ or LAG-3−cells from WT or KO TxT donors were i.v. injected into diabetic C57BL/6 recipients 1 day prior to transplantation with BALB/c pancreatic islets. WT LAG-3+, black line with closed circle (n = 12); KO LAG-3+, black line with closed square (n = 6), WT LAG-3−, black dashed line with open circle (n = 7); and KO LAG-3−, black dashed line with open square (n = 3). (e) CD4+LAG-3+ or LAG-3−cells from TxT mice were injected into C57BL/6 recipients of pancreatic islets from unrelated (C3H) donors. Experimental groups are the same as in (d), WT LAG-3+ (n = 5); KO LAG-3+ (n = 3) and WT LAG-3− (n = 5). (f) CD4+LAG-3+ cells from UM WT and Ptpn22 −/− donors were injected into C57BL/6 recipients of BALB/c pancreatic islets. WT LAG-3+, black line with closed circle (n = 4); and KO LAG-3+, black line with closed square (n = 2). Graft rejection was monitored over time. **p < 0.01, ***p < 0.001; NS, not statistically significant
To address whether PTPN22 regulates Tr1 cell function, the potency of LAG-3+ cells from Ptpn22 −/− and WT TxT mice was compared in adoptive transfer experiments. Upon injection into newly transplanted mice (BALB/c → C57BL/6), splenic CD4+LAG-3+ T cells from Ptpn22 −/− TxT mice transferred transplant tolerance, similar to Tr1 cells from WT TxT mice (Fig. 5d). LAG-3−CD4+ splenocytes from Ptpn22 −/− TxT mice also delayed allograft rejection (Fig. 5d). CD4+LAG-3+ T cells from Ptpn22 −/− TxT mice controlled allograft rejection in an antigen-specific manner, since recipients of islets from an irrelevant donor (i.e. C3H → C57BL/6) rejected the graft (Fig. 5e). CD4+LAG-3+ T cells isolated from UM Ptpn22 −/− mice did not confer protection from graft rejection (Fig. 5f), demonstrating that CD4+LAG3+ T cells from TxT mice are enriched in Tr1 cells that mediate transplant tolerance in an antigen-specific manner.
Lack of PTPN22 strengthens transplant tolerance
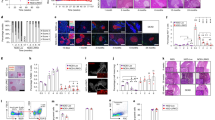
To test whether the spleen plays a key role in maintaining transplant tolerance in Ptpn22 −/− mice, as it does in WT mice [30, 31], splenectomy was performed. Splenectomised Ptpn22 −/− TxT mice rejected grafts with kinetics similar to those of WT mice (Fig. 6a), thus confirming the importance of the spleen in this transplant tolerance model [30, 31].
Transplant tolerance after G-CSF/RAPA treatment requires splenic Tr1 cells only in WT mice, while both Tr1 and Treg cells are required to maintain transplant tolerance in Ptpn22 −/− mice. Tolerance was established in WT and Ptpn22 −/− (KO) Tx mice with G-CSF/RAPA. (a) Following a boost, TxT mice underwent splenectomy. WT, black line with circles (n = 6); KO, grey line with squares (n = 6). (b) An anti-LAG-3 mAb was administered to TxT mice. WT, black line with circles (n = 11); KO, grey line with squares (n = 7). Some KO TxT mice that did not reject after the first anti-LAG-3 infusion were treated with a ×5 greater dose of anti-LAG-3. (c) Concomitant administration of anti-LAG-3 mAb and P60 peptide in TxT mice. WT, black line with circle (n = 3); KO, grey line with squares (n = 6). Rejection kinetics is shown by group. (d, e) Mean fluorescent intensity (MFI) for LFA-1 and CTLA-4 on FOXP3+ CD4+ T cells from WT and KO TxT mice was evaluated 100 days after transplantation. Histogram overlays depict the expression levels of LFA-1 and CTLA-4 for representative WT and Ptpn22 −/− TxT mice. Grey filled line on the left indicates negative staining. Black numbers indicate MFI on FOXP3+ T cells from WT TxT mice, and grey numbers from KO TxT mice. Graphs in (e) show results from WT (open circle) and KO (open square) TxT mice ± SEM. *p < 0.05, ***p < 0.001; NS, not statistically significant
Next, the requirement for Tr1 cells in maintaining transplant tolerance in Ptpn22 −/− mice was tested by administering an anti-LAG-3 mAb. Whereas anti-LAG-3 mAb treatment inhibited transplant tolerance in WT TxT mice, Ptpn22 −/− TxT mice remained tolerant (Fig. 6b). Simultaneous P60 peptide and anti-LAG-3 mAb administration inhibited transplant tolerance in Ptpn22 −/− TxT mice and accelerated graft rejection in WT TxT mice (Fig. 6b, c). These results show that both Tr1 and Treg cells mediate transplant tolerance in Ptpn22 −/− mice, while tolerance in WT mice is predominantly mediated by Tr1 cells. To address whether Treg cells are more potent in Ptpn22 −/− TxT than in WT TxT mice, the expression of phenotypic markers linked to Treg cell function was measured. Treg cells from Ptpn22 −/− TxT mice expressed higher levels of LFA-1 and CTLA-4, but similar levels of programmed death-1 (PD-1), CD103, CD44, CD62L and Ki-67 (Fig. 6d, e and data not shown) compared with those of WT TxT mice, suggesting they have increased function, in agreement with previous reports [19]. In conclusion, a lack of PTPN22 further stabilises G-CSF/RAPA-mediated transplant tolerance by promoting FOXP3+ Treg and Tr1 cells that act in synergy in vivo.
Discussion
A genetic variation affecting PTPN22 (R620W) is one of the major non-HLA genetic risk factors for autoimmunity. Whereas several mouse and human studies have shown that PTPN22*W increases adaptive and dampens innate immune responses [1, 2], it remains unclear how it predisposes to autoimmunity. Here, we show in a mouse model of pancreatic islet transplantation that alloreactive T cell numbers are increased in the spleens of Ptpn22 −/− mice but are controlled by the compensatory expansion of FOXP3+ Treg and LAG-3+ Tr1 cells. Ptpn22 −/− transplanted mice treated with G-CSF/RAPA develop robust transplant tolerance, which is maintained via both Tr1 and Treg cells. This contrasts with WT mice, in which mainly Tr1 cells sustain transplant tolerance after the same tolerogenic treatment [30]. These data show that a lack of PTPN22 strengthens transplant tolerance in mice and suggests that PTPN22 may serve to therapeutically target Treg and Tr1 cells.
The resistance of Ptpn22 −/− mice to develop spontaneous autoimmunity despite heightened lymphoproliferation and abnormal expansion of memory T cells was attributed to the enhanced development of FOXP3+ Treg cells [19, 21]. We found increased Treg cell thymic development in the absence of PTPN22, similar to a previous study [21] in which the same knockout mouse line was used. This contrasts with another study in which different knockout mice were used and no difference in Treg thymic development was seen [19]. The reasons for this discrepancy are unclear.
Our study provides new data supporting the hypothesis that Tr1 cells expand in Ptpn22 −/− mice and might have an ancillary function in protection from autoimmunity. The mechanism by which PTPN22 controls the number and function of Tr1 and Treg cells is not completely understood. It is possible that PTPN22 controls Tr1 and Treg development by modulating the threshold of T cell activation, as originally suggested [7]. Alternatively, it may act by modulating the number, tissue distribution and function of professional antigen-presenting cells (APCs), known to play a key role in Treg cell development and function [36–38].
We previously showed that whereas PTPN22 sets the threshold of Treg cell conversion in vitro, it affects the accumulation/survival, and to a lesser extent the differentiation, of Treg cells in vivo [39]. In the present study, Treg and Tr1 cell numbers were already increased in the spleens of Ptpn22 −/− mice, and their expansion after G-CSF/RAPA treatment was similar to that of WT mice. Thus, PTPN22 does not interfere with the expansion/induction of Treg and Tr1 cells induced by G-CSF/RAPA treatment.
Treg cells were found at a higher frequency in the spleen and in kidney dLNs of Ptpn22 −/− TxT mice than of WT TxT mice, but were present at similar levels in the graft. In contrast, Tr1 cells were found at a higher frequency in the spleens of Ptpn22 −/− TxT mice than of WT TxT mice, while their frequency was similar in kidney dLNs and they were almost absent from the graft. These data confirm our previous observations by showing that transplant tolerance induced via G-CSF/RAPA treatment is maintained in WT mice by Tr1 cells that predominantly reside in the spleen [30, 31]. However, transplant tolerance in Ptpn22 −/− mice is maintained by both Tr1 and Treg cells that act in both the spleen and kidney dLNs.
Treg and Tr1 cells suppress target cells via various mechanisms, some of which are shared, e.g. the killing or modulation of APCs and the production of IL-10 [40]. A previous study showed that Ptpn22 −/− Treg cells produce increased amounts of IL-10 in the steady state compared with WT cells and have enhanced adhesive properties due to increased levels of LFA-1 [19]. We found that Ptpn22 −/− Treg cells from tolerant mice express higher levels of LFA-1 and CTLA-4 than WT cells but produce similar levels of IL-10. Treg depletion or blockade was insufficient to inhibit transplant tolerance in Ptpn22 −/− mice, whereas LAG-3 blockade inhibited transplant tolerance only in WT mice. When FOXP3 and LAG-3 blockade were combined, tolerance was lost in Ptpn22 −/− mice and graft rejection was accelerated in WT mice. This result shows that Treg cells maintain transplant tolerance in Ptpn22 −/− mice in the absence of Tr1 cells, suggesting that the increased expression of LFA-1 and CTLA-4 on Treg cells makes them more potent in vivo.
Tr1 cells from tolerant mice transferred transplant tolerance in an antigen-specific manner comparable to that of WT mice. Interestingly, while control LAG-3− T cells from WT TxT mice did not transfer tolerance, LAG-3− T cells from TxT Ptpn22 −/− mice conferred a transient tolerogenic effect. These data suggest that a fraction of LAG-3− Ptpn22 −/− T cells was regulatory (i.e. FOXP3+) or acquired regulatory function in vivo after adoptive transfer.
Taken together, our data show that PTPN22 plays a key role in Treg and Tr1 cell generation, with important implications for transplant tolerance. Lack of PTPN22 enhances the number of Treg and Tr1 cells in the steady state and strengthens transplant tolerance by increasing the regulatory properties of Treg cells. Treg and Tr1 cells in tolerant mice migrate to different sites, as we previously showed [31], and act synergistically or operate via overlapping mechanisms. Together, these findings suggest that PTPN22 can serve to therapeutically target Treg and Tr1 cells to moderate immune responses toward allografts.
Abbreviations
- APC:
-
Antigen-presenting cell
- CTLA-4:
-
Cytotoxic T-lymphocyte protein 4
- dLN:
-
Draining lymph node
- FOXP3:
-
Forkhead box protein P3
- G-CSF:
-
Granulocyte-colony stimulating factor
- LAG-3:
-
Lymphocyte activation gene 3
- LFA-1:
-
Lymphocyte function-associated antigen 1
- mAb:
-
Monoclonal antibody
- PD-1:
-
Programmed death-1
- PE:
-
Phycoerythrin
- PTPN22:
-
Protein tyrosine phosphatase non-receptor 22
- pTreg:
-
Peripherally-derived Treg
- RAPA:
-
Rapamycin
- TCR:
-
T cell receptor
- TDS:
-
T cell-depleted splenocytes
- Tr1:
-
T regulatory type 1
- Treg:
-
T regulatory
- Tx:
-
Mice transplanted with BALB/c islets and receiving no treatment
- TxT:
-
Mice transplanted with BALB/c islets and considered tolerant
- UM:
-
Unmanipulated
- WT:
-
Wild-type
References
Bottini N, Peterson EJ (2013) Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol 32:83–119
Stanford SM, Bottini N (2014) PTPN22: the archetypal non-HLA autoimmunity gene. Nat Rev Rheumatol 10:602–611
Fousteri G, Liossis SN, Battaglia M (2013) Roles of the protein tyrosine phosphatase PTPN22 in immunity and autoimmunity. Clin Immunol 149:556–565
Stanford S, Mustelin T, Bottini N (2010) Lymphoid tyrosine phosphatase and autoimmunity: human genetics rediscovers tyrosine phosphatases. Semin Immunopathol 32:127–136
Gjorloff-Wingren A, Saxena M, Williams S, Hammi D, Mustelin T (1999) Characterization of TCR-induced receptor-proximal signaling events negatively regulated by the protein tyrosine phosphatase PEP. Eur J Immunol 29:3845–3854
Wu DJ, Zhou W, Enouz S et al (2014) Autoimmunity-associated LYP-W620 does not impair thymic negative selection of autoreactive T cells. PLoS One 9:e86677
Hasegawa K (2004) PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 303:685–689
Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R (2014) The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol 15:875–883
Arechiga AF, Habib T, He Y et al (2009) Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol 182:3343–3347
Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner J (2007) Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol 179:4704–4710
Habib T, Funk A, Rieck M et al (2012) Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol 188:487–496
Wang Y, Shaked I, Stanford SM et al (2013) The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity 39:111–122
Bottini N, Musumeci L, Alonso A et al (2004) A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 36:337–338
Bottini N, Vang T, Cucca F, Mustelin T (2006) Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol 18:207–213
Criswell LA, Pfeiffer KA, Lum RF et al (2005) Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet 76:561–571
Zhang J, Zahir N, Jiang Q et al (2011) The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet 43:902–907
Aarnisalo J, Treszl A, Svec P et al (2008) Reduced CD4+T cell activation in children with type 1 diabetes carrying the PTPN22/Lyp 620Trp variant. J Autoimmun 31:13–21
Vang T, Congia M, Macis M et al (2005) Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet 37:1317–1319
Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R (2012) Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal 5:ra87
Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A (2009) PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol 182:4093–4106
Maine C, Hamilton-Williams E, Cheung J et al (2012) PTPN22 alters the development of regulatory T cells in the thymus. J Immunol 188:5267–5275
Chang HH, Miaw SC, Tseng W et al (2013) PTPN22 modulates macrophage polarization and susceptibility to dextran sulfate sodium-induced colitis. J Immunol 191:2134–2143
Maine CJ, Marquardt K, Cheung J, Sherman LA (2014) PTPN22 controls the germinal center by influencing the numbers and activity of t follicular helper cells. J Immunol 192:1415–1424
Zheng P, Kissler S (2013) PTPN22 silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant. Diabetes 62:896–904
Yeh LT, Miaw SC, Lin MH et al (2013) Different modulation of Ptpn22 in effector and regulatory T cells leads to attenuation of autoimmune diabetes in transgenic nonobese diabetic mice. J Immunol 191:594–607
Li XC, Turka LA (2010) An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol 6:577–583
Curotto de Lafaille M, Lafaille J (2009) Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30:626–635
Gagliani N, Magnani CF, Huber S et al (2013) Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 19:739–746
Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK (2006) Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 212:28–50
Gagliani N, Gregori S, Jofra T et al (2011) Rapamycin combined with anti-CD45RB mAb and IL-10 or with G-CSF induces tolerance in a stringent mouse model of islet transplantation. PLoS One 6:e28434
Gagliani N, Jofra T, Valle A et al (2013) Transplant tolerance to pancreatic islets is initiated in the graft and sustained in the spleen. Am J Transplant 13:1963–1975
Fousteri G, Dave A, Bot A, Juntti T, Omid S, von Herrath M (2010) Subcutaneous insulin B:9−23/IFA immunisation induces Tregs that control late-stage prediabetes in NOD mice through IL-10 and IFNgamma. Diabetologia 53:1958–1970
Casares N, Rudilla F, Arribillaga L et al (2010) A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J Immunol 185:5150–5159
Huang CT, Workman CJ, Flies D et al (2004) Role of LAG-3 in regulatory T cells. Immunity 21:503–513
Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM (2007) Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol 178:4136–4146
Ezzelarab M, Thomson AW (2011) Tolerogenic dendritic cells and their role in transplantation. Semin Immunol 23:252–263
Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W (2014) Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol 5:7
Chung CY, Ysebaert D, Berneman ZN, Cools N (2013) Dendritic cells: cellular mediators for immunological tolerance. Clin Dev Immunol 2013:972865
Fousteri G, Jofra T, Debernardis I et al (2014) The protein tyrosine phosphatase PTPN22 controls forkhead box protein 3 T regulatory cell induction but is dispensable for T helper type 1 cell polarization. Clin Exp Immunol 178:178–189
Gregori S, Goudy KS, Roncarolo MG (2012) The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol 3:30
Acknowledgements
We would like to thank all current and previous members of M. Battaglia’s and MG Roncarolo’s laboratory at the San Raffaele Research Institute. We are grateful to the staff of the flow cytometry and animal facilities and to Juan José Lasarte and Noelia Casares from the Departamento de Medicina Interna, Facultad de Medicina, Universidad de Navarra, Pamplona, Spain, for providing the P60 peptide. Ptpn22 −/− mice were obtained from S Rahmouni (University of Liege, Belgium) after a material transfer agreement with Genentech (San Francisco, California, USA).
Funding
This work was supported by a Giovani Ricercatori 2007 grant (D.lgs 502/92) from the Italian Ministry of Health to MB and the Marie Curie Reintegration Grant FP7 PEOPLE to GF (276745). GF is supported by a JDRF advanced postdoctoral fellowship (10-2014-204).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author contributions
GF, MB and NG conceived and designed experiments; and GF, TJ, RDF, NG, CM and AS performed the experiments and acquired and analysed the data. GF and MB wrote the manuscript and supervised the study. All authors critically read and revised the manuscript and approved its final version.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 401 kb)
ESM Fig. 2
(PDF 228 kb)
ESM Fig. 3
(PDF 187 kb)
ESM Fig. 4
(PDF 62 kb)
ESM Fig. 5
(PDF 73 kb)
Rights and permissions
About this article
Cite this article
Fousteri, G., Jofra, T., Di Fonte, R. et al. Lack of the protein tyrosine phosphatase PTPN22 strengthens transplant tolerance to pancreatic islets in mice. Diabetologia 58, 1319–1328 (2015). https://doi.org/10.1007/s00125-015-3540-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-015-3540-9