Abstract
Aims/hypothesis
Reduced beta cell mass due to increased beta cell apoptosis is a key defect in type 2 diabetes. Islet amyloid, formed by the aggregation of human islet amyloid polypeptide (hIAPP), contributes to beta cell death in type 2 diabetes and in islet grafts in patients with type 1 diabetes. In this study, we used human islets and hIAPP-expressing mouse islets with beta cell Casp8 deletion to (1) investigate the role of caspase-8 in amyloid-induced beta cell apoptosis and (2) test whether caspase-8 inhibition protects beta cells from amyloid toxicity.
Methods
Human islet cells were cultured with hIAPP alone, or with caspase-8, Fas or amyloid inhibitors. Human islets and wild-type or hIAPP-expressing mouse islets with or without caspase-8 expression (generated using a Cre/loxP system) were cultured to form amyloid. Caspase-8 and -3 activation, Fas and FLICE inhibitory protein (FLIP) expression, islet beta cell and amyloid area, IL-1β levels, and the beta:alpha cell ratio were assessed.
Results
hIAPP treatment induced activation of caspase-8 and -3 in islet beta cells (via Fas upregulation), resulting in apoptosis, which was markedly reduced by blocking caspase-8, Fas or amyloid. Amyloid formation in cultured human and hIAPP-expressing mouse islets induced caspase-8 activation, which was associated with Fas upregulation and elevated islet IL-1β levels. hIAPP-expressing mouse islets with Casp8 deletion had comparable amyloid, IL-1β and Fas levels with those expressing hIAPP and Casp8, but markedly lower beta cell apoptosis, higher beta:alpha cell ratio, greater beta cell area, and enhanced beta cell function.
Conclusions/interpretation
Beta cell Fas upregulation by endogenously produced and exogenously applied hIAPP aggregates promotes caspase-8 activation, resulting in beta cell apoptosis. The prevention of amyloid-induced caspase-8 activation enhances beta cell survival and function in islets.
Similar content being viewed by others
Introduction
Reduced beta cell mass resulting from increased beta cell apoptosis is a key defect in type 2 diabetes [1, 2]. Islet amyloid formation in patients with type 2 diabetes contributes to progressive beta cell death [3–5]. Unlike in type 2 diabetes, amyloid forms rapidly in cultured [6–8] and transplanted human islets [9, 10], and this is associated with beta cell dysfunction and death in vitro [6–8], and with islet graft failure leading to recurrence of hyperglycaemia in animal models of type 1 diabetes [10–12]. Moreover, amyloid deposition associated with reduced beta cell mass has been reported in islet grafts in patients with type 1 diabetes [13, 14]. Thus islet amyloid, in addition to its role in the pathogenesis of type 2 diabetes, also contributes to islet graft failure in patients with type 1 diabetes.
Islet amyloid polypeptide (amylin), the major component of islet amyloid, is a neuroendocrine hormone that is co-localised and co-secreted with insulin from beta cells in response to beta cell secretagogues [15]. Three major factors contribute to human islet amyloid polypeptide (hIAPP) aggregation in type 2 diabetes: (1) presence of an amyloidogenic sequence in the hIAPP molecule [4]; (2) elevated beta cell production and secretion of hIAPP due to increased insulin demand [16, 17]; and (3) impaired prohIAPP processing and/or trafficking due to beta cell dysfunction [17–19]. Impaired clearance of secreted hIAPP because of disrupted blood vessels in isolated islets may also potentiate amyloid formation. Islet amyloid is typically found extracellular, adjacent to beta cells [6–8, 13, 20, 21], but intracellular hIAPP aggregates also form in humans and transgenic rodent models [9, 13, 18, 21]. Small hIAPP aggregates appear to be the major mediators of beta cell death [3, 4, 22].
In vitro studies have suggested various mechanisms for amyloid-induced beta cell death, including: the formation of non-selective ion-channel-like structures [23]; interaction of (pro)hIAPP with components of beta cell membranes (e.g. heparan sulphate proteoglycans) [24]; inducing endoplasmic reticulum stress [25] and oxidative stress [26]; and disruption of the endoplasmic reticulum-associated degradation (ERAD)/ubiquitin/proteasome system [27] and autophagy/lysosomal pathway [28]. Although several mechanisms, at least in vitro, are involved in hIAPP-induced beta cell apoptosis, some of these may share the same apoptotic signalling pathways.
Fas-mediated apoptosis has been implicated in beta cell death in type 2 diabetes [29, 30]. This pathway is initiated by interaction between the cell death receptor Fas (CD95/APO-1) and the Fas ligand (FasL/CD95L), which recruits caspase-8 (FLICE) to the death-inducing signalling complex (DISC) through interaction with the Fas-associated death domain adaptor protein. Cleaved (active) caspase-8 in turn activates downstream caspases, mainly caspase-3 [31]. Moreover, caspase-8 can indirectly activate caspase-9, the key enzyme in the cytochrome c (mitochondrial) apoptotic pathway, by its cleavage of BH3 interacting domain, which in turn evokes release of cytochrome c from mitochondria [32]. The major endogenous regulator of Fas-mediated apoptosis is the cellular FLICE inhibitory protein (FLIP), which prevents cleavage of procaspase-8 and subsequent apoptotic signals by its recruitment and cleavage in the DISC instead of procaspase-8 [31].
Islet beta cells constitutively express FasL but do not normally express Fas at detectable levels [7, 29]. However, exposure to metabolic stress such as elevated glucose [29], fatty acids [33], leptin [34] or cytokines (e.g. IL-1β) [30, 35] induces beta cell Fas upregulation. We recently showed that amyloid formation in human and hIAPP transgenic mouse islets induces Fas upregulation in beta cells [7]. In this study, we used primary beta cells and human islets to examine whether amyloid-induced Fas upregulation in islet beta cells can promote activation of caspase-8, the key enzyme in the Fas-mediated apoptotic pathway. We also generated a mouse model with beta cell specific hIAPP expression and Casp8 deletion, to test whether blockade of caspase-8 can protect beta cells from the hIAPP aggregates formed in islets.
Methods
Human islet isolation and culture
Human islets isolated from cadaveric pancreatic donors (36–54 years old) were provided by the Ike Barber Human Islet Transplant Laboratory (Vancouver, BC, Canada) in accordance with approved procedures and guidelines of the Clinical Research Ethics Board of the University of British Columbia. Hand-picked human islets (purity >90%, dithizone staining) were cultured in CMRL (Mediatech, Herndon, VA, USA) supplemented with 11.1 mmol/l glucose, 10% (vol./vol.) FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and 50 μg/ml gentamicin. Islets were cultured in a humidified 5% CO2/95% air incubator (37°C, 7 days) and the medium was replaced every 2 days.
Animal models
Transgenic hemizygous C57BL/6 mice with beta cell hIAPP expression (hIAPP +/−) were kindly provided by S. Kahn (University of Washington, Seattle, WA, USA) and maintained by cross-breeding with DBA/2J mice (Jackson Laboratory, Bar Harbor, ME, USA). Male hIAPP +/− mice on a high-fat diet form islet amyloid and develop amyloid-associated diabetes in about 1 year [20], whereas isolated islets from hIAPP +/− mice form amyloid within days during culture with elevated glucose [7, 19, 36]. Mice with beta cell-specific Casp8 deletion (RIPcre + Casp8 fl/fl) were generated from Casp8 fl/fl mice [37] using the Cre/loxP recombinase system [38] and maintained by inter-breeding RIPcre + Casp8 fl/+ mice. Homozygous mice have age-dependent (8–12 months) defects in beta cell mass in the presence of enhanced insulin secretion [38].
To generate mice with beta cell-specific hIAPP expression and Casp8 deletion, hIAPP +/− and RIPcre + Casp8 fl/fl mice were cross-bred to produce hIAPP +/−/RIPcre + Casp8 fl/+ mice, which then were inter-bred to generate hIAPP +/RIPcre + Casp8 fl/fland hIAPP +/RIPcre + Casp8 +/+ mice. The presence of the hIAPP transgene [39], insulin-cre transgene [40] and disrupted caspase-8 gene [37] was determined. The first generation of these offspring (10–28 weeks) was used for studies. Animals were fed mouse chow containing 9% (wt/wt) fat (Purina 5021; LabDiet, Richmond, IN, USA). Animals were cared for in accordance with the Guidelines and Principles of Laboratory Animal Care, and the standard procedures established by the Canadian Council on Animal Care and the University of British Columbia’s Animal Policy and Welfare Committee.
Mouse islet isolation
Mice were anaesthetised with tribromoethanol (0.25 mg/g body weight, i.p.) and killed by cervical dislocation. Ice-cold collagenase (1,000 U/ml, type XI; Sigma-Aldrich, Oakville, ON, Canada) in 2 ml calcium-free Hanks’ buffer was injected via the common bile duct. Pancreases were removed and incubated for 14 min with additional 2 ml collagenase (1,000 U/ml in Hanks’ buffer) in a water bath at 37°C, followed by gentle shaking for 2 min. Digestion was stopped by adding ice-cold Hanks’ buffer containing 1 mmol/l CaCl2. Digested tissues were rinsed, centrifuged (250 g, 30 s, 4°C) and resuspended in the same solution, then filtered through a 70 μm mesh cell strainer (BD Biosciences, Oakville, ON, Canada) into Ham’s-F10 (Invitrogen, Burlington, ON, Canada). To allow recovery, hand-picked islets (purity >95%) were cultured overnight in Ham’s-F10 supplemented with 10 mmol/l glucose, 0.5% (wt/vol.) BSA and antibiotics as detailed for human islets, and then cultured for 7 days in Ham’s-F10 with 16.7 mmol/l glucose.
Islet dissociation, culture and treatments
Human or mouse islets were dissociated as described [41] and cultured in poly-l-lysine-coated 8-well chamber slides in CMRL (human) or Ham’s-F10 (mouse) with 5.5 and 10 mmol/l glucose, respectively. Lyophilised hIAPP and rat islet amyloid polypeptide (rIAPP) aliquots were prepared from synthetic peptides (1–37 aa; Bachem, Torrance, CA, USA) as before [41], dissolved fresh in culture medium and added to islet cells at a final concentration of 10 μmol/l. The amyloid inhibitor Congo red (Sigma; dissolved in dimethyl sulfoxide), the caspase-8 inhibitor (z-LETD-FMK; Bachem) or the Fas antagonist (Kp7-6; EMD Chemicals, Gibbstown, NJ, USA) were prepared in culture medium at final concentrations of 25 μmol/l, 100 μmol/l and 10 mmol/l, respectively, and added to cells 1 h before hIAPP treatment.
Immunolabelling of cells and islets
Islet cells were fixed in 4% paraformaldehyde (wt/vol.) and permeabilised with Triton X-100. Paraffin-embedded islet sections (4 μm) were dewaxed, rehydrated and blocked in 2% normal goat or donkey serum (Vector Laboratories, Burlingame, CA, USA). Cells or islet sections (following antigen retrieval with citrate buffer) were incubated overnight at 4°C with guinea pig anti-insulin (1:1,000; Dako, Carpinteria, CA, USA) and each of the following: rabbit anti-glucagon (1:750; Dako), cleaved (active) caspase-8 or -3 (1:100; Cell Signaling, Pickering, ON, Canada), precursor caspase-8 (1:50; Santa Cruz, Santa Cruz, CA, USA), Fas (1:50; Santa Cruz), oligomer (A11) (1:400; Invitrogen), IL-1β (1:100; Santa Cruz) or proliferating cell nuclear antigen (PCNA) (1:250; Cell Signaling). They were then washed with PBS, incubated for 1 h at room temperature with Texas red-conjugated anti-guinea pig (Jackson Laboratories, West Grove, PA, USA) and Alexa 488-conjugated anti-rabbit or anti-mouse (Molecular Probes, Eugene, OR, USA), and counterstained with the nuclear dye DAPI (blue; Vector Laboratories) for quantification studies or with 7-aminoactinomycin D (red; Molecular Probes). For insulin and A11 staining, Alexa 488-conjugated anti-guinea pig (Molecular Probes) and Texas red-conjugated anti-rabbit (Jackson) were used as secondary antibodies, respectively. For TUNEL staining, after immunolabelling for insulin, islet sections (or cells) were incubated (30 min, 37°C) with TUNEL reaction mixture (1:20; Roche Diagnostics, Laval, QC, Canada) and for amyloid staining with 0.5% (wt/vol.) thioflavin S solution (5 min, room temperature) (Sigma).
Islet insulin and hIAPP content and release
Mouse islets (25 per condition) were pre-incubated (1 h, 37°C) in KRB buffer containing 10 mmol/l HEPES (pH 7.4), 0.25% BSA (wt/vol.) and 1.67 mmol/l glucose, followed by 1 h incubation in KRB containing 1.67 mmol/l glucose (basal insulin release) and another hour in KRB containing 16.7 mmol/l glucose (stimulated insulin release). Islets were lysed in 100 μl of 1 mol/l acetic acid/0.1% BSA (10 min, 100°C). Insulin levels were measured by a mouse-specific insulin ELISA kit (Alpco Diagnostics, Salem, NH, USA). Islet hIAPP content and release were assessed by a human amylin (total) ELISA kit (EZHAT-51K; EMD Millipore, Billerica, MA, USA). All values were normalised to islet protein levels (Pierce/Thermo Scientific, Rockford, IL, USA).
Results
Exposure to exogenous hIAPP induces caspase-8 activation in human islet beta cells, which is associated with Fas upregulation and precedes caspase-3 activation and apoptosis
Human islet cells were cultured in normal glucose with or without synthetic hIAPP or non-fibrillogenic rIAPP at different time points (4–24 h). Non-treated and rIAPP-treated islet cells had very low numbers of beta cells positive for active caspase-8 (Fig. 1a, Electronic supplementary material [ESM] Fig. 1a). Treatment with hIAPP induced caspase-8 activation in islet beta cells in a time-dependent manner. The maximum number of beta cells positive for active caspase-8 was detectable by immunolabelling between 8 and 12 h after exposure to hIAPP (Fig. 1a). The time point of caspase-8 activation (8–12 h) correlated with that of Fas upregulation (8–12 h), and preceded caspase-3 activation (16 h) and beta cell apoptosis (24 h) in hIAPP-treated islet beta cells (Fig. 1b–d, ESM Fig. 1b).
Exposure to exogenous hIAPP induces caspase-8 activation and apoptosis in human islet beta cells, which is associated with Fas upregulation. (a) Islet cells were immunolabelled for insulin and active caspase-8 (aCasp8) following 8 or 12 h of treatment with hIAPP or rIAPP. (b) Immunolabelling for insulin and Fas, insulin and active caspase-3 (aCasp3), or insulin and TUNEL as indicated after 12, 16 or 24 h of hIAPP treatment, respectively. Scale bar = 100 μm; inserts: (a) ×2.6; (b) ×3.5. (c) The proportion of beta cells positive for active caspase-3 and (d) TUNEL in hIAPP-treated and non-treated cells. Quantifications represent a minimum of ten microscopic fields each containing 100 to 150 cells. Results are expressed as means ± SEM of four independent studies performed in triplicate; *p < 0.05 by Student’s t test
Blocking hIAPP-mediated caspase-8 activation by amyloid, caspase-8 or Fas inhibitors markedly reduces the beta cell apoptosis induced by the aggregation of exogenously applied hIAPP
To identify the mechanisms underlying amyloid-induced caspase-8 activation, we tested the effects of the amyloid inhibitor Congo red, the Fas antagonist and the caspase-8 inhibitor on hIAPP-treated human islet beta cells. The amyloid-binding dye Congo red significantly reduced the number of active caspase-8 and apoptotic beta cells in hIAPP-treated cells (Fig. 2a–c). Similarly, blockade of Fas (induced by hIAPP) with a Fas antagonist and inhibition of caspase-8 both markedly lowered the number of active caspase-8-positive and apoptotic beta cells in hIAPP-treated islet cells (Fig. 2a–c). Basal death in untreated islet beta cells was comparable with that in cells treated with Congo red alone, but was slightly lower in those treated with the Fas antagonist or caspase-8 inhibitor alone (Fig. 2c). Finally, hIAPP-induced beta cell apoptosis was markedly lower in islet cells from RIPcre + Casp8 fl/fl mice lacking caspase-8 than in wild-type mice that expressed caspase-8 (Fig. 2d, e).
Blockade of hIAPP-mediated caspase-8 activation markedly reduces beta cell apoptosis induced by aggregation of exogenously applied hIAPP. Human islet cells were treated for 8, 12 or 24 h with hIAPP alone or with each of the following: the amyloid inhibitor Congo red (CR), a caspase-8 inhibitor (Casp8 inh) or a Fas antagonist (Kp7-6). (a) Immunolabelling for insulin (red), active caspase-8 (aCasp8; green) and DAPI (blue). Scale bar = 10 μm. (b) The proportion of beta cells positive for active caspase-8 and (c) TUNEL. (d) Immunolabelling for insulin and active caspase-8, with (e) the proportion of TUNEL-positive beta cells in RIPcre + Casp8 fl/fl and wild-type (RIPcre + Casp8 +/+) mouse islet cells treated with hIAPP for 8 or 24 h, respectively. Scale bar = 50 μm. Quantifications represent a minimum of ten microscopic fields each containing 100 to 150 islet cells. Results are expressed as means ± SEM of three independent studies performed in triplicate; *p < 0.05 by one-way ANOVA
Amyloid formation in human islets during culture induces caspase-8 activation and beta cell death, which is associated with Fas upregulation
We examined whether the formation of biosynthetic hIAPP aggregates in human islets during culture can induce caspase-8 activation in beta cells. Isolated human islets from six donors were cultured for 7 days in elevated glucose to potentiate amyloid formation. Thioflavin S (amyloid)-positive islets were not detectable in freshly isolated islets from five of six donors, but small amyloid-positive areas were detectable in about 1% of islets from one donor (Fig. 3a–c). Islet culture resulted in progressive amyloid formation in all human islet preparations. Similarly, oligomer (A11)-positive islets were detectable in cultured islets from all donors (Fig. 3a, d). Importantly, amyloid formation in cultured human islets was associated with caspase-8 activation in beta cells. Furthermore, thioflavin S- or oligomer (A11)-positive areas in islets closely correlated with active caspase-8-positive beta cell areas, both of which were associated with increased beta cell apoptosis (Fig. 3a, e) and reduced islet beta cell area (Fig. 3a, f).
The formation of biosynthetic hIAPP aggregates in human islets during culture induces caspase-8 activation and beta cell apoptosis, which is associated with Fas upregulation. (a) Human islets at days 0 and 7 of culture were immunolabelled as indicated for: insulin, active caspase-8 (aCasp8) and thioflavin S (Thio S), or inserts: insulin (green) and A11 for smaller hIAPP aggregates (red); insulin and Fas; insulin and TUNEL; and insulin and glucagon. Scale bar = 50 μm; inserts: ×2 (A11: ×3). The proportion of (b) thioflavin S (amyloid)-positive islets to the total number of islets, (c) amyloid area to total islet area, (d) oligomer (A11)-positive islets to the total number of islets, (e) TUNEL-positive beta cells and (f) beta cell area to total islet area. Results are expressed as means ± SEM of six independent studies; n = 25–30 islets per condition in each study; *p < 0.05 by Student’s t test
The deletion of Casp8 in hIAPP-expressing mouse islet beta cells markedly reduces apoptosis induced by the aggregation of biosynthetic hIAPP during culture
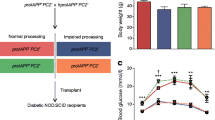
We generated a mouse model with beta cell-specific hIAPP expression and Casp8 deletion (Fig. 4), to test whether blockade of caspase-8 activation can protect beta cells from amyloid toxicity in an ex vivo model of human islet amyloid formation. During 7 days of culture, hIAPP-expressing mouse islets formed amyloid, which was detectable by insulin/thioflavin S and insulin/oligomer (A11) immunolabelling (Figs 4c, 5a). Cultured islets from hIAPP-expressing mice with or without Casp8 deletion had comparable levels of amyloid formation, hIAPP content and release (Figs 4c, 5c–g).
Beta cell-specific deletion of Casp8 in hIAPP-expressing mouse islets. (a) hIAPP transgene, disrupted Casp8 and insulin-cre transgene were detected by PCR performed on tail sample DNA from 3-week-old mice. Results for wild-type littermates with or without Casp8 deletion and that do not express hIAPP are shown for comparison. (b) Pancreatic sections from 8-week-old hIAPP +/RIPcre + Casp8 fl/fl and hIAPP +/RIPcre + Casp8 +/+ mice immunolabelled for insulin and full-length caspase-8 (Casp8). The white dashed line outlines the islet in the pancreatic section. (c) Islet sections from each genotype immunolabelled for insulin with thioflavin S (Thio S) and nuclear staining. Scale bar = 50 μm; inserts: ×1.7
Amyloid formation and amyloid-induced caspase-8 activation in hIAPP-expressing mouse islets with or without beta cell-specific Casp8 deletion. (a) Islets from wild-type and hIAPP-expressing mice with or without beta cell caspase-8 deletion were immunolabelled following 7 days culture as indicated for insulin and active caspase-8 (aCasp8); insulin and thioflavin S (Thio S); and insulin and A11 for smaller hIAPP aggregates. Scale bar = 50 μm; inserts: ×1.5 (A11: ×3). The proportion of (b) active caspase-8-positive beta cells (fold over wild-type), (c) thioflavin S (amyloid)-positive islets to the total number of islets, (d) amyloid area to total islet area and (e) oligomer (A11)-positive islets to the total number of islets. (f) Islet hIAPP release and (g) content were assessed in cultured islets from hIAPP-expressing mice with or without Casp8 deletion (wild-type taken as 100%). Quantifications represent 17–25 islets per animal from each genotype (six to nine mice per group). Results are expressed as means ± SEM; *p < 0.05 by Student’s t test
Similarly to human islets, the aggregation of biosynthetic hIAPP in transgenic mouse islets during culture induced activation of caspase-8 (Fig. 5a, b). A few active caspase-8-positive beta cells were present in cultured islets from wild-type mice expressing caspase-8, but such cells were not detectable in wild-type or hIAPP-expressing islets with beta cell Casp8 deletion (Fig. 5a). Also, a small number of active caspase-8-positive non-beta cells were present in all genotypes. Amyloid formation and amyloid-induced Fas upregulation were associated with elevated islet IL-1β immunoreactivity in cultured hIAPP-expressing mouse islets with and without Casp8 deletion (Fig. 6a, b). There was no detectable difference between FLIP levels in islet lysates from hIAPP-expressing and wild-type mice (Fig. 6c). Despite similar islet IL-1β and beta cell Fas levels, hIAPP-expressing mouse islets lacking caspase-8 had significantly lower rates of beta cell apoptosis during culture than islets expressing hIAPP and caspase-8 (Fig. 7a, b), resulting in higher beta:alpha cell ratios and islet beta cell areas in those islets (Fig. 7d, e). Finally, the beta cell proliferation rate was not significantly different in the four genotypes (Fig. 7c).
Fas and IL-1β immunoreactivity, and FLIP protein levels in hIAPP-expressing mouse islets with or without beta cell-specific Casp8 deletion. (a) Paraffin-embedded sections of 7-day cultured islets from wild-type and hIAPP-expressing mice with or without Casp8 deletion were immunolabelled as indicated for insulin, Fas and thioflavin S (Thio S), or insulin, IL-1β and thioflavin S. Scale bar = 50 μm; inserts: ×1.5. (b) The proportion of Fas-positive islet beta cells (fold over wild-type: hIAPP −/−/Casp8 +/+). (c) FLIP protein levels in lysates (15 μg) from 7-day cultured wild-type and hIAPP-expressing mouse islets (12–14 weeks) using anti-FLIP antibody (1:500; Santa Cruz). Quantifications represent 20–25 islets per animal from each genotype (five to six mice per group). Results are expressed as means ± SEM; *p < 0.05 by one-way ANOVA. FLIPL, long form FLIP; GAPDH, glyceraldehyde-3-phosphate dehydrogenase
Beta cell-specific deletion of Casp8 in hIAPP-expressing mouse islets markedly reduces apoptosis induced by aggregation of biosynthetic hIAPP during culture. (a) Islets from wild-type and hIAPP-expressing mice with or without Casp8 deletion were immunolabelled following 7 days culture as indicated for insulin and TUNEL, or insulin and glucagon. Scale bar = 50 μm; inserts: ×1.5. (b) The proportion of apoptotic and (c) proliferating islet beta cells, (d) the islet beta:alpha cell ratio and (e) beta cell area per total islet area in each genotype. Quantifications represent 17–25 islets per animal from each genotype (six to nine mice per group). Results are expressed as means ± SEM; *p < 0.05 by one-way ANOVA
Cultured hIAPP-expressing mouse islets with Casp8 deletion have enhanced beta cell function compared with those expressing Casp8
Increased beta cell apoptosis in cultured islets from hIAPP-expressing mice was associated with reduced insulin response to elevated glucose (stimulation index) and reduced insulin content compared with wild-type littermates (Fig. 8). The stimulation index was 32% higher in cultured islets from hIAPP-expressing mice lacking caspase-8 than in islets expressing hIAPP and caspase-8, a finding that correlated with the higher (36%) insulin content in those islets (Fig. 8). Cultured islets from mice with Casp8 deletion and without hIAPP expression had a greater (15%) insulin response to elevated glucose and higher (20%) insulin content than their wild-type littermates. However, this increase was not as profound as the difference observed between hIAPP-expressing mice lacking or expressing caspase-8, suggesting that the deletion of Casp8 improves the beta cell dysfunction caused by amyloid formation in hIAPP-expressing mouse islets during culture.
Cultured hIAPP-expressing mouse islets with Casp8 deletion have enhanced beta cell function compared with those expressing caspase-8. (a) Insulin response to glucose stimulation and (b) insulin content in islets from wild-type and hIAPP-expressing mice with or without Casp8 deletion following 7 days culture. Glucose-stimulated insulin release (stimulation index) indicates the amount of insulin secreted during 1 h of incubation at 1.67 (basal) and 16.7 mmol/l (stimulated) glucose. Insulin content is reported as per cent of the insulin content in wild-type islets, which was set at 100%. Results are expressed as means ± SEM of five to six animals per genotype; *p < 0.05 by one-way ANOVA
Discussion
We recently showed that hIAPP aggregates induce Fas expression in islet beta cells [7], which do not normally express Fas at detectable levels [7, 29]. In the present study, using human islet beta cells and two ex vivo models of human islet amyloid formation, we provide direct evidence that amyloid-induced Fas upregulation in islet beta cells can promote activation of caspase-8, the key mediator of Fas apoptotic signalling, thereby initiating apoptosis. We also show that the deletion of Casp8 protects islet beta cells from the cytotoxic effects of biosynthetic hIAPP aggregates formed in islets.
In human islet beta cells (but not alpha cells), aggregates of exogenously applied hIAPP induced activation of caspase-8 and -3, resulting in apoptosis, a process that was prevented by amyloid, caspase-8 or Fas inhibitors. Caspase-8 activation preceded caspase-3 activation and apoptosis, and followed Fas upregulation. These findings suggest that Fas upregulation induced by hIAPP aggregates promotes interaction between Fas and FasL on neighbouring cells (beta cells, other islet or non-islet cells), thereby inducing caspase-8 activation. Consistent with our findings in human islet beta cells, a previous in vitro study showed that synthetic hIAPP induces caspase-8 activation in rodent beta cells [42]. Interestingly, aggregates of amyloid beta peptide were also shown to induce caspase-8 activation and apoptosis in neural cells, probably via the Fas-mediated apoptotic pathway [43]. Moreover, active caspase-8 and Fas-positive cells have been reported in the brain in Alzheimer’s disease [44, 45]. Thus, it seems likely that hIAPP aggregates in type 2 diabetes and amyloid beta aggregates in Alzheimer’s disease share the same apoptotic signalling pathway(s).
The formation of biosynthetic hIAPP aggregates in human and hIAPP-expressing mouse islets during culture was associated with caspase-8 activation in beta cells, leading to increased beta cell apoptosis and reduced beta cell mass. Interestingly, despite comparable levels of amyloid, IL-1β and Fas in cultured hIAPP-expressing mouse islets with or without caspase-8 expression, islets lacking caspase-8 had markedly lower beta cell apoptosis, a higher beta:alpha cell ratio and a greater islet beta cell area than islets expressing caspase-8. Moreover, deletion of Casp8 in hIAPP-expressing mouse islets enhanced beta cell function, suggesting that the inhibition of caspase-8 can reduce the beta cell toxicity and improve the beta cell dysfunction mediated by biosynthetic hIAPP aggregates.
The majority of islets with amyloid formation were positive for IL-1β, Fas and caspase-8 immunoreactivity, whereas islets with no detectable amyloid formation had very low levels of all three, suggesting that amyloid may induce Fas upregulation and caspase-8 activation by promoting IL-1β release in islets. In support of this, hIAPP aggregates have been shown to promote maturation of proIL-1β to IL-1β via activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome [46, 47]. In addition, we recently showed that the inhibition of amyloid formation reduces islet IL-1β levels and Fas upregulation [7]. The cell source(s) of IL-1β in amyloid-positive islets could be beta cells, non-beta or non-islet cells (e.g. macrophages). Both macrophage-positive and macrophage-negative islet areas have been reported in hIAPP transgenic mouse islets [46]. It seems therefore that macrophages are the major, but possibly not the only source of amyloid-induced IL-1β production in islets. Taken together, these findings suggest that amyloid formation may contribute to islet inflammation in patients with type 2 diabetes [48].
The balance between caspase-8 and its cellular regulator FLIP can switch Fas-mediated signalling to apoptosis or proliferation [49, 50]. This raises the possibility that hIAPP aggregates, like elevated glucose, may reduce beta cell FLIP levels, thereby switching Fas signalling towards apoptosis. Although we did not detect any significant difference between FLIP protein levels in lysates from 7-day islet cultures from hIAPP-expressing and wild-type mice, it is possible that potential effects of hIAPP aggregates on beta cell FLIP levels were masked in our experimental model for the following reasons: (1) elevated glucose (used to potentiate amyloid formation) can increase FLIP levels in wild-type and hIAPP-expressing mouse islets; and (2) FLIP protein levels were measured in lysates from islet cultures that contained amyloid-positive and -negative islets.
In summary, our studies show that beta cell Fas upregulation induced by hIAPP aggregates is probably mediated via release of IL-1β from islets and promotes Fas and FasL interaction, thereby initiating Fas-mediated apoptosis. We also show that deletion of Casp8 protects islet beta cells from amyloid toxicity, suggesting that caspase-8 plays a significant role in amyloid-induced beta cell apoptosis.
Abbreviations
- DISC:
-
Death-inducing signalling complex
- FasL:
-
Fas ligand
- FLIP:
-
FLICE inhibitory protein
- hIAPP:
-
Human islet amyloid polypeptide
- PCNA:
-
Proliferating cell nuclear antigen
- rIAPP:
-
Rat islet amyloid polypeptide
References
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110
Hanley SC, Austin E, Assouline-Thomas B et al (2010) Beta-cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology 151:1462–1472
Haataja L, Gurlo T, Huang CJ, Butler PC (2008) Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev 29:303–316
Westermark P, Andersson A, Westermark GT (2011) Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 91:795–826
Jurgens CA, Toukatly MN, Fligner CL et al (2011) Beta-cell loss and beta-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol 178:2632–2640
Marzban L, Tomas A, Becker TC et al (2008) Small interfering RNA-mediated suppression of proislet amyloid polypeptide expression inhibits islet amyloid formation and enhances survival of human islets in culture. Diabetes 57:3045–3055
Park YJ, Lee S, Kieffer TJ et al (2012) Deletion of Fas protects islet beta cells from cytotoxic effects of human islet amyloid polypeptide. Diabetologia 55:1035–1047
Park YJ, Ao Z, Kieffer TJ et al (2013) The glucagon-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: implications in type 2 diabetes and islet transplantation. Diabetologia 56:508–519
Westermark G, Westermark P, Eizirik DL et al (1999) Differences in amyloid deposition in islets of transgenic mice expressing human islet amyloid polypeptide versus human islets implanted into nude mice. Metabolism 48:448–454
Potter KJ, Abedini A, Marek P et al (2010) Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci U S A 107:4305–4310
Udayasankar J, Kodama K, Hull RL et al (2009) Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia 52:145–153
Westwell-Roper C, Dai DL, Soukhatcheva G et al (2011) IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol 187:2755–2765
Westermark GT, Westermark P, Berne C, Korsgren O (2008) Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med 359:977–979
Westermark GT, Davalli AM, Secchi A et al (2012) Further evidence for amyloid deposition in clinical pancreatic islet grafts. Transplantation 93:219–223
Kahn SE, D'Alessio DA, Schwartz MW et al (1990) Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes 39:634–638
Hou X, Ling Z, Quartier E et al (1999) Prolonged exposure of pancreatic beta cells to raised glucose concentrations results in increased cellular content of islet amyloid polypeptide precursors. Diabetologia 42:188–194
Zheng X, Ren W, Zhang S et al (2010) Serum levels of proamylin and amylin in normal subjects and patients with impaired glucose regulation and type 2 diabetes mellitus. Acta Diabetol 47:265–270
Paulsson JF, Andersson A, Westermark P, Westermark GT (2006) Intracellular amyloid-like deposits contain unprocessed pro-islet amyloid polypeptide (proIAPP) in beta cells of transgenic mice overexpressing the gene for human IAPP and transplanted human islets. Diabetologia 49:1237–1246
Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB (2006) Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes 55:2192–2201
Verchere CB, D'Alessio DA, Palmiter RD et al (1996) Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A 93:3492–3496
Janson J, Soeller WC, Roche PC et al (1996) Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A 93:7283–7288
Gurlo T, Ryazantsev S, Huang CJ et al (2010) Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol 176:861–869
Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280:17294–17300
Park K, Verchere CB (2001) Identification of a heparin binding domain in the N-terminal cleavage site of pro-islet amyloid polypeptide. Implications for islet amyloid formation. J Biol Chem 276:16611–16616
Huang CJ, Lin CY, Haataja L et al (2007) High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56:2016–2027
Zraika S, Hull RL, Udayasankar J et al (2009) Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 52:626–635
Costes S, Huang CJ, Gurlo T et al (2011) Beta-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes 60:227–238
Rivera JF, Gurlo T, Daval M et al (2011) Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic beta-cells: protective role of p62-positive cytoplasmic inclusions. Cell Death Differ 18:415–426
Maedler K, Spinas GA, Lehmann R et al (2001) Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 50:1683–1690
Maedler K, Sergeev P, Ris F et al (2002) Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860
Lavrik IN, Krammer PH (2012) Regulation of CD95/Fas signaling at the DISC. Cell Death Differ 19:36–41
Kaufmann T, Strasser A, Jost PJ (2012) Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ 19:42–50
Boni-Schnetzler M, Boller S, Debray S et al (2009) Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150:5218–5229
Maedler K, Sergeev P, Ehses JA et al (2004) Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A 101:8138–8143
Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11:98–107
Zraika S, Hull RL, Udayasankar J et al (2007) Glucose- and time-dependence of islet amyloid formation in vitro. Biochem Biophys Res Commun 354:234–239
Salmena L, Lemmers B, Hakem A et al (2003) Essential role for caspase 8 in T cell homeostasis and T cell-mediated immunity. Genes Dev 17:883–895
Liadis N, Salmena L, Kwan E et al (2007) Distinct in vivo roles of caspase-8 in beta-cells in physiological and diabetes models. Diabetes 56:2302–2311
Andrikopoulos S, Verchere CB, Terauchi Y, Kadowaki T, Kahn SE (2000) Beta-cell glucokinase deficiency and hyperglycemia are associated with reduced islet amyloid deposition in a mouse model of type 2 diabetes. Diabetes 49:2056–2062
Postic C, Shiota M, Niswender KD et al (1999) Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274:305–315
Law E, Lu S, Kieffer TJ et al (2010) Differences between amyloid toxicity in alpha and beta cells in human and mouse islets and the role of caspase-3. Diabetologia 53:1415–1427
Zhang S, Liu H, Yu H, Cooper GJ (2008) Fas-associated death receptor signaling evoked by human amylin in islet beta-cells. Diabetes 57:348–356
Wei W, Norton DD, Wang X, Kusiak JW (2002) Abeta 17-42 in Alzheimer's disease activates JNK and caspase-8 leading to neuronal apoptosis. Brain J Neurol 125:2036–2043
Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH (2001) Activation of caspase-8 in the Alzheimer's disease brain. Neurobiol Dis 8:1006–1016
Yew DT, Ping Li W, Liu WK (2004) Fas and activated caspase 8 in normal, Alzheimer and multiple infarct brains. Neurosci Lett 367:113–117
Masters SL, Dunne A, Subramanian SL et al (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol 11:897–904
Westwell-Roper C, Dunne A, Kim ML, Verchere CB, Masters SL (2013) Activating the NLRP3 inflammasome using the amyloidogenic peptide IAPP. Methods Mol Biol 1040:9–18
Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M (2013) Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab 17:860–872
Maedler K, Fontana A, Ris F et al (2002) FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc Natl Acad Sci U S A 99:8236–8241
Maedler K, Schumann DM, Sauter N et al (2006) Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes 55:2713–2722
Acknowledgements
Human islets for these studies were provided by the Ike Barber Human Islet Transplant Laboratory (Vancouver, BC, Canada). Human IAPP transgenic mice were kindly provided by S. Kahn (VA Puget Sound Health Care System, University of Washington, Seattle, WA, USA). We gratefully acknowledge the outstanding technical contribution of I. Barta (Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada), A. Asadi and T. Webber (Department of Cellular and Physiological Sciences, University of British Columbia, Vancouver, BC, Canada) to the completion of these studies.
Funding
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to LM (MOP-126204) and MW (MOP-81148). Infrastructure support was provided by a grant from the Canadian Foundation for Innovation (LM). YJP is the recipient of a studentship from the CIHR Transplant Research Training Program.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors contributed to the study conception and design, or the analysis and interpretation of data, as well as to the drafting of the article or its critical revision for important intellectual content. All authors gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 1.13 MB)
Rights and permissions
About this article
Cite this article
Park, Y.J., Woo, M., Kieffer, T.J. et al. The role of caspase-8 in amyloid-induced beta cell death in human and mouse islets. Diabetologia 57, 765–775 (2014). https://doi.org/10.1007/s00125-013-3152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3152-1