Abstract
Aims/hypothesis
Increased inflammation and oxidative stress are associated with insulin resistance (IR) and metabolic disorders. Serum histidine levels are lower and are negatively associated with inflammation and oxidative stress in obese women. The objective of this study was to evaluate the efficacy of histidine supplementation on IR, inflammation, oxidative stress and metabolic disorders in obese women with the metabolic syndrome (MetS).
Methods
A total of 100 obese women aged 33–51 years with BMI ≥ 28 kg/m2 and diagnosed with MetS were included following a health examination in the community hospital in this randomised, double-blinded, placebo-controlled trial. Participants were allocated to interventions by an investigator using sequentially numbered sealed envelopes and received 4 g/day histidine (n = 50) or identical placebo (n = 50) for 12 weeks. Participants then attended the same clinic every 2 weeks for scheduled interviews and to count tablets returned. Serum histidine, HOMA-IR, BMI, waist circumference, fat mass, serum NEFA, and variables connected to inflammation and oxidative stress were measured at baseline and 12 weeks. Participants, examining physicians and investigators assessing the outcomes were blinded to group assignment. In addition, the inflammatory mechanisms of histidine were also explored in adipocytes.
Results
At 12 weeks, a total of 92 participants completed this trail. Compared with the placebo group (n = 47), histidine supplementation significantly decreased HOMA-IR (−1.09 [95% CI −1.49, −0.68]), BMI (−0.86 kg/m2 [95% CI −1.55, −0.17]), waist circumference (−2.86 cm [95% CI −3.86, −1.86]), fat mass (−2.71 kg [95% CI −3.69, −1.73]), serum NEFA (−173.26 μmol/l [95% CI −208.57, −137.94]), serum inflammatory cytokines (TNF-α, −3.96 pg/ml [95% CI −5.29, −2.62]; IL-6, −2.15 pg/ml [95% CI −2.52, −1.78]), oxidative stress (superoxide dismutase, 17.84 U/ml [95% CI 15.03, 20.65]; glutathione peroxidase, 13.71 nmol/ml [95% CI 9.65, 17.78]) and increased serum histidine and adiponectin by 18.23 μmol/l [95% CI 11.74, 24.71] and 2.02 ng/ml [95% CI 0.60, 3.44] in histidine supplementation group (n = 45), respectively. There were significant correlations between changes in serum histidine and changes of IR and its risk factors. No side effects were observed during the intervention. In vitro study indicated that histidine suppresses IL6 and TNF mRNA expression and nuclear factor kappa-B (NF-κB) protein production in palmitic acid-induced adipocytes in a dose-dependent manner, and these changes were diminished by an inhibitor of NF-κB.
Conclusions/interpretation
Histidine supplementation could improve IR, reduce BMI, fat mass and NEFA and suppress inflammation and oxidative stress in obese women with MetS; histidine could improve IR through suppressed pro-inflammatory cytokine expression, possibly by the NF-κB pathway, in adipocytes.
Trial registration
www.chictr.org/cn/ChiCTR-TRC-11001551
Funding
The study was supported by the National Natural Science Fund of China (No. 81202184, 81130049, 81102112), Heilongjiang Post/doctoral Fund (No. LBN-Z12193) and Key Laboratory of Nutrition and Food Hygiene (Harbin Medical University, Heilongjiang Higher Education Institutions, No. YYKFKT1202).
Similar content being viewed by others
Introduction
Insulin resistance (IR) is the main feature and common root for the metabolic syndrome (MetS), type 2 diabetes and cardiovascular disease [1–3]. Dietary intervention in IR has been reported to be effective in reducing the risks for these chronic diseases, especially type 2 diabetes [4]. Inflammation and oxidative stress are known to play a central role in the occurrence and aggression of IR [2, 5, 6]; suppressing low-grade chronic inflammation and oxidative stress could reduce the severity IR and reduce the risks for type 2 diabetes and cardiovascular disease [2, 5, 7]. Therefore, clinicians and public health researchers have become interested in the search for components from foods that have activity against inflammation and oxidative-stress.
Histidine is an important amino acid for humans. It has been reported that low plasma concentrations of histidine are associated with protein-energy wasting, inflammation, oxidative stress and increased mortality in patients with chronic kidney disease [8]. Further, in a previous study we found that a lower serum histidine level in obese women, compared with non-obese healthy women, was associated with inflammation and oxidative stress [9]. Moreover, another recent study also found that the histidine level was lower in young obese individuals and in individuals with type 2 diabetes and was correlated with insulin sensitivity [10].
Based on the hypothesis that histidine may improve insulin sensitivity and alleviate inflammation, oxidative stress and metabolic disorders in humans, we performed this randomised, double-blinded, placebo-controlled trial in obese women with MetS and also carried out an in vitro study in adipocytes to clarify this issue.
Methods
Participants
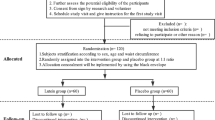
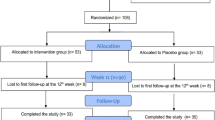
A total of 100 obese women with MetS, aged 33–51 years, BMI ≥ 28 kg/m2 [11], were recruited from five communities near Harbin Medical University via posters for this trial. Of the 303 obese women who attended the health examination, 151 were excluded for being diagnosed as not having MetS and 52 refused to participate, finally resulting in 100 individuals participating in this trial (Fig. 1).
MetS was defined according to the International Diabetes Federation criteria [12] as waist circumference (WC) ≥80 cm for women plus at least two of the following components: (1) raised triacylglycerol (TG) (>1.7 mmol/l); (2) reduced HDL-cholesterol (<1.29 mmol/l); (3) raised systolic blood pressure (SBP) (≥130 mmHg) or diastolic blood pressure (DBP) (≥85 mmHg); (4) hyperglycaemia (fasting blood glucose [FBG] level of ≥5.6 mmol/l or 2 h postload blood glucose [2 h-PG] of ≥7.8 mmol/l). The exclusion criteria were: (1) unstable body weight during the past 6 months (change in BMI >0.5 kg/m2); (2) myocardial infarction, injuries, chronic or acute inflammatory diseases, taking anti-inflammatory medication or pregnancy/lactation; (3) kidney, liver or gastrointestinal diseases; (4) recent initiation of exercise programme; (5) recent initiation/change in hormonal birth control or hormone replacement therapy. This study was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving humans were approved by the Human Research Ethics Committee of the Harbin Medical University. Written informed consent was obtained from all participants.
Intervention
Histidine powder was purchased from Yuan Cheng Gong Chuang (Wu Han, China). The l-histidine (purity 99.65%) was obtained from microbial fermentation and was food grade and safe for human use. Histidine was produced, using maize starch, into tablets, each containing 1 g histidine. An identical placebo was produced, just containing maize starch.
Before the intervention, a pilot experiment was designed to determine the optimal dose of histidine supplementation. According to the histidine intake and supplementation described in previous studies [13–16], we recruited 20 obese female university students for the pilot experiment, randomly assigned into two groups: ten women took two 1 g histidine tablets after breakfast and two tablets after dinner (4 g/day histidine) and the other ten women took four tablets after breakfast and four tablets after dinner (8 g/day histidine) for 2 weeks. During the supplementation period, no side effects were reported and serum superoxide dismutase (SOD) was increased in both groups at the second week (data not shown). Thus, we selected histidine 4 g/day as the intervention dose.
For the trial, 100 participants were randomly assigned to one of two groups, and received either histidine or placebo. To ensure the homogeneity of outcome variables at baseline across the placebo and histidine supplementation groups, the homogeneity of age, BMI and serum histidine at baseline were tested. If the homogeneity of the outcome variables at baseline was not satisfied, then a new set of random numbers was generated from a randomisation number table and participants were randomised again by one of the authors (C. Zhao), who also labelled the treatment/placebo-containing bottles and dispensed them to participants.
During the intervention, the histidine supplementation group received 4 g/day histidine: two 1 g histidine tablets after breakfast and two tablets after dinner for 12 weeks; the placebo group received four tablets of placebo daily. The compliance of the study participants was assessed by scheduled interviews and counting tablets returned every 2 weeks. After the routine interview, a 2-week supply of placebo or histidine tablets (four tablets/day × 2 weeks = 56 tablets) were given to each participant. The participants and the field staff responsible for data collection and outcome measures all remained blinded to the allocation of the two groups during the entire intervention.
Demographic characteristics
Demographic data were collected at baseline using a standardised questionnaire. Information collected included age, physical activity, current smoking, alcohol use, health history, medications and menstrual status The level of physical activity during leisure time was defined as: 0 = none, 1 = 1 ∼ 30 min/week, 2 = 31 ∼ 60 min/week, 3 = 61 ∼ 90 min/week, 4 = 91 ∼ 120 min/week and 5 ≥ 120 min/week. The level of physical activity at work was coded as: 1 = sedentary, 2 = moderate and 3 = heavy.
Dietary intake
At baseline and the 12th week, dietary intakes were measured with a validated semi-quantitative food-frequency questionnaire (FFQ) based on frequency [17]. Energy and nutrient intakes were calculated using the Food Nutrition Calculator (V1.60; Shixinghengxun, Beijing, China).
Anthropometric and laboratory measurements
Anthropometric indices were measured by well-trained examiners with participants wearing light, thin clothing and no shoes. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. Blood pressure was measured three times, using a standard mercury sphygmomanometer and appropriate sizes of adult cuffs on the right arm, after a 10 min rest in a sitting position. BMI was calculated as weight (kg) divided by the square of height (metres). WC was measured midway between the lowest rib and the iliac crest using a flexible anthropometric tape in the horizontal plane with the participant in a standing position, measured to the nearest 0.1 cm. Fat mass (FM) was measured using the electric impedance method with a body fat mass analyser (ioi 353; Janex Medical, Seoul, Korea).
Participants were instructed to fast for 12 h and fasting blood samples were collected in the morning. Then, each participant was given an oral glucose tolerance test and blood samples were drawn 2 h after the 75 g glucose was taken. Antecubital venous blood was centrifuged to obtain serum and stored at −80°C.
Serum histidine was determined as previously described [9]. Briefly, samples of serum (100 μl) were spiked with acetonitrile (100 μl), vortex-mixed for 30 s, immediately centrifuged at 11,000 g for 10 min at 4°C and the supernatant fraction was recovered. An Alliance 2695 separation module and a 2487 ultraviolet detector from Waters were used for the HPLC analysis (CV 4.2–5.3%). Derivatisation and gradient elution of serum amino acids were carried out following the manufacturer’s instructions (Waters, Taunton, MA, USA).
ELISA using commercial kits was carried out, according to the manufacturers’ instructions, for assay of histamine (IBL, Hamburg, Germany), C-reactive protein (CRP) (Biocheck, Foster City, CA, USA), TNF-α (R&D Systems Europe, Abingdon, UK), IL-6 (R&D Systems Europe) and adiponectin (R&D Systems Europe). SOD, malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) were measured with commercial kits using enzymatic methods (Jiancheng Technology, Nanjing, China).
FBG, 2 h-PG, total cholesterol (TC), HDL-cholesterol, LDL-cholesterol, TG, NEFA, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), serum creatinine (Crea), blood urea nitrogen (BUN) and serum uric acid (UA) were determined using a ROCHE Modular P800 Automatic Biochemical Analyzer (Roche Diagnostics, Mannheim, Germany). Serum insulin concentration was measured by ROCHE Elecsys 2010 Chemiluminescence Immune Analyzer (Roche Diagnostics). Intra- and inter-day CV% of the clinical variables is shown in the electronic supplementary material (ESM Methods). The HOMA-IR index was calculated as previously described [18].
In vitro study
Histidine (99% purity), oleic acid (OA) (99% purity) and palmitic acid (PA) (99% purity) were purchased from Sigma-Aldrich (St Louis, MO, USA). HEPES and BSA were from Amersco (Solon, OH, USA). Secondary antibody and quinazoline (QNZ) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Akt, p-Akt, NF-κB/p65 and β-actin antibodies were from Cell signalling technology (Danvers, MA, USA).
To prepare fatty acids, PA (25 mmol/l) was dissolved in NaOH (50 mmol/l) at 70°C and mixed with 10% (wt/vol.) BSA at 55°C, as 5 mmol/l reserving liquid. The reserving liquid was then diluted 1:10 (vol./vol.) with DMEM/F-12 (3:1) to obtain a 0.5 mmol/l PA solution with which to induce IR and inflammation. Histidine was dissolved in PBS and diluted to different concentrations (0, 1, 5 and 10 mmol/l) with serum-free DMEM for treatment.
For cell culture we used the SW872 cell line (American Tissue Culture Collection, Rockville, MD, USA) that had been used in our previous study [19], cultured in DMEM/F-12 (3:1) containing 10% FBS and 1% penicillin/streptomycin in 5% CO2/95% air at 37°C [20]. Confluent monolayers of pre-adipocytes were induced to differentiate according to the method we previous used [19].
To study the effect of histidine on IR and inflammation, after serum starvation, SW872 cells were treated with 0.5 mmol/l PA, with or without histidine and nuclear factor kappa-B (NF-κB)/p65 inhibitor for 24 h, stimulated with or without 100 nmol/l insulin for 10 min, and harvested. Histidine was added into the medium to concentrations of 0, 1, 5 and 10 mmol/l, and NF-κB/p65 inhibitor was added (QNZ, 10 nmol/l) [21]. Then phosphorylated protein kinase B (pAkt)/Akt and NF-κB/p65 protein and gene expressions (IL6 and TNF mRNA) were examined.
Total RNA was isolated from cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed with the SYBR Green PCR Master Mix and a 7500 FAST Real-time PCR System (Applied Biosystems). The primer sequences are listed in ESM Table 1.
For western blot analysis, nuclear protein was extracted using a commercial kit (Beyotime Institute of Biotechnology, Beijing, China) for NF-κB/p65. The detailed protocol for western blot in our laboratory has been described in a previous study [19]. Experiments were replicated at least three times and a representative blot was shown.
Statistical analysis
The calculation of statistical power was performed before the commencement of the study. At 80% statistical power and 5% significance level, a sample size of 32 in each group will be sufficient to detect a mean difference of 10 U/ml of SOD with an SD of 14 between the supplementation and the placebo groups. At 90% statistical power, a sample size of 43 per group will be needed. Statistical analyses were carried out using SAS software (version 9.1; SAS Institute, Cary, NC, USA). Data were presented as mean ± SD or percentage. The χ 2 test was used to compare categorical variables. Paired samples t test was used to evaluate the changes in outcome variables before and after intervention in each group. Mean levels of continuous study variables at baseline and the 12th week between the two groups were also compared using analysis of covariance (ANCOVA). In the model of ANCOVA, the covariates included baseline values, age, serum histidine, protein intake, physical activity, alcohol use, current smoking and menopause. The percentage differences between supplementation and placebo groups in outcome variables at the 12th week were calculated using covariate-adjusted values as 100 × ([histidine supplementation group–placebo group]/placebo group) for each study variable. Partial correlation analyses were conducted to examine the relationship between changes of serum histidine and changes of IR and its risk factors, adjusted for age, serum histidine, protein intake, physical activity, alcohol use, current smoking and menopause age, serum histidine, protein intake, physical activity, alcohol use, current smoking and menopause. In the in vitro study, differences among the groups were determined by ANOVA followed by post hoc multiple comparison tests. All p values were two-tailed and p < 0.05 was considered significant for statistical analyses.
Results
Retention and compliance of participants
Figure 1 shows the study design and the flow of participants. Among the 100 eligible individuals who participated in the study at baseline, 92 completed the trial and took all the tablets. Five individuals in the placebo group were excluded from analysis because they changed residence (n = 1), did not take the suggested quantity of tablets (n = 3) or were lost to follow-up (n = 1). Three individuals in the histidine supplementation group were excluded because they did not take the suggested quantity of tablets (n = 2) or became pregnant (n = 1). There were no significant differences in the overall compliance rates between the two groups (90% in placebo group, 94% in histidine supplementation group, p = 0.46).
Characteristics of the participants
At baseline, there were no significant differences between the placebo and histidine supplementation groups in age, dietary factors, physical activity, percentage of current smoking, alcohol use, menopause, anthropometric or clinical variables (Tables 1, 2). Dietary nutrient intakes did not differ significantly across the two groups at either baseline or end of the trial, or the changes in dietary nutrient intakes during the intervention period were not significant within each group, suggesting that histidine did not suppress appetite at the dose used (ESM Table 2). None of the participants complained of headache, weakness, drowsiness or nausea during the intervention.
Effect of histidine supplementation on IR, obesity index, serum histidine, histamine and other metabolic variables
Mean levels (±SD) of variables at baseline and the 12th week in the two groups are presented in Table 2. There were no significant differences in variables between the two groups at baseline. After 12 weeks of intervention, there was a significant increase in serum histidine (18.23 μmol/l [95%CI 11.74, 24.71]) and adiponectin (2.02 ng/ml [95% CI 0.60, 3.44]), and a significant decrease in HOMA-IR (−1.09 [95% CI −1.49, −0.68]), BMI (−0.86 kg/m2 [95% CI −1.55, −0.17]), WC (−2.86 cm [95% CI −3.86, −1.86]), FM (−2.71 kg [95% CI −3.69, −1.73]) and NEFA (−173.26 μmol/l [95% CI −208.57, −137.94]) in the histidine supplementation group compared with baseline; the change in serum histamine was not significant. However, FBG showed a decreased trend in the intervention group (−0.8 mmol/l, p = 0.052). In the placebo group, there were no significant changes in these outcome variables measured over the intervention period.
Figure 2 shows the percentage differences between the histidine supplementation and placebo groups at the 12th week, adjusted for baseline values, age, serum histidine, protein intake, physical activity, current smoking, alcohol use and menopause status in the ANCOVA analyses. Histidine supplementation, compared with placebo, significantly decreased the HOMA-IR, BMI, WC, FM and NEFA by 18.9%, 2.9%, 3.7%, 6.0% and 18.1%, respectively, and increased serum histidine and adiponectin by 13.2% and 21.5%, respectively. Change in TG did not reach significance and FBG was decreased in trend by ANCOVA analyses.
Effect of histidine supplementation on low-grade inflammation and oxidative stress
There were no significant differences between the histidine supplementation and placebo group in inflammation and oxidative stress markers at baseline (Table 3). After 12 weeks of intervention, histidine supplementation significantly increased serum SOD (17.84 U/ml [95% CI 15.03, 20.65]) and GSH-Px (13.71 nmol/ml [95% CI 9.65, 17.78]) and decreased TNF-α (−3.96 pg/ml [95% CI −5.29, −2.62]) and IL-6 (−2.15 pg/ml [95% CI −2.52, −1.78]) compared with baseline. In the placebo group, no significant changes in these outcome variables were observed over the intervention period.
When compared with placebo, histidine significantly decreased inflammatory markers (TNF-α, −28.3%; IL-6, −29.3%) and improved oxidative stress (SOD, 16.1% and GSH-Px, 9.0%) at the 12th week (Fig. 2), which indicated that histidine could suppress inflammation and oxidative stress.
Effect of histidine supplementation on liver and kidney variables
There were no significant differences in values of serum AST, ALT, GGT, ALP, Crea, BUN or UA over the intervention period or between the two groups (ESM Table 3), which indicated that histidine did not influence the liver or kidney functions.
Partial correlations between change in serum histidine and HOMA-IR and IR risk factors
Partial correlations were conducted between the change in serum histidine and HOMA-IR, IR risk factors (Table 4). The data showed that the change in serum histidine was significantly correlated with changes in HOMA-IR, NEFA, TNF-α, IL-6, SOD, GSH-Px, FM, WC and BMI, with or without the adjustment for age and serum histidine, protein intake, physical activity, alcohol use, current smoking and menopause at baseline.
In vitro study of the effect of histidine on IR and inflammation
After the SW872 adipocytes were treated with 0.5 mmol/l PA for 24 h to induce IR and inflammation, the expression of pAkt/Akt was significantly attenuated (Fig. 3a). Histidine treatment increased pAkt/Akt in a dose-dependent manner, while QNZ, an NF-κB inhibitor, diminished the effects of histidine and PA. These results showed that PA reduced insulin sensitivity in adipocytes; histidine could improve IR and this effect depended on NF-κB.
Effect of histidine on IR and inflammation induced by PA in human adipocytes. (a) pAkt/Akt ratio evaluated as densitometric analysis of western blot bands in whole cell lysates from normal control or PA-induced cells at different doses of histidine. (b) mRNA levels of IL6 in differently treated cells determined by real-time PCR. (c) mRNA levels of TNF in differently treated cells determined by real-time PCR. (d) Nuclear NF-κB evaluated as densitometric analysis of western blot bands in differently treated cells. Cells were seeded at a density of 200,000 cells/ml in each flask. Mature cells were grown for 24 h in DMEM/F-12 (10% FBS), washed with PBS and incubated with or without PA and different concentrations of histidine (0, 1, 5, 10 mmol/l) in DMEM/F-12 for 24 h and with or without QNZ (10 nmol/l) for 2 h. After these treatments, cells were washed twice with PBS and lysates were prepared. Results are expressed as a percentage of the corresponding control cells. Bars represent mean values in triplicate. * p < 0.05, ** p < 0.01 compared with the IR and inflammatory model; INS, insulin; HIS, histidine. † p < 0.05, †† p < 0.01 compared with the control (significance was assessed using one-way ANOVA, followed by Dunnett’s multiple comparison test)
Further, we investigated the effect of histidine on markers of inflammation. PA could stimulate the NF-κB pathway to induce inflammation; IL6 and TNF mRNA expression and NF-κB protein production in adipocytes were significantly increased after incubating cells with PA indicating that inflammation was induced (Fig. 3b–d). After incubation with histidine 0, 1, 5 and 10 mmol/l for 24 h, IL6 and TNF mRNA expression and NF-κB protein expression were decreased in a dose-dependent manner, suggesting that histidine could improve the inflammation status in adipocytes. When pre-incubated with NF-κB inhibitor, we found that IL6 and TNF mRNA expression were significantly lower than in the inflammatory group; addition of histidine did not improve the IL6 and TNF mRNA expression or NF-κB production. Our findings indicate that histidine could improve IR and inflammation in adipocytes, probably through the NF-κB pathway.
Discussion
This randomised, double-blind, placebo-controlled trial found that histidine supplementation for 12 weeks significantly decreased HOMA-IR, BMI, WC, FM, NEFA and the inflammatory biomarkers of TNF-α and IL-6, and increased serum histidine, adiponectin, SOD and GSH-Px, suggesting that histidine could improve IR and fat accumulation and suppress inflammation and oxidative stress in obese women with MetS.
As the data for histidine supplementation are limited, we first considered the dose of histidine supplementation. As a large dose of histidine (64 g/day) was reported to induce side effects of headache, weakness, drowsiness and nausea [22], and a low dose may not produce the significant effects we expected, we conducted a pilot experiment with 4 g/day and 8 g/day histidine supplementation to determine an effective and safe dose. We found that both doses could significantly increase SOD at week 2, with no side effects or suppressed appetite. For the sake of safety, we selected 4 g/day for this study. In this 12-week intervention, we did not observe any side effects or changes in liver enzymes or kidney variables. In addition, although serum histidine increased after the supplementation, we did not see any change in serum histamine or receive complaints of suppressed appetite, headache, weakness, drowsiness, or nausea in either the group receiving 4 g/day histidine or the group receiving placebo, which was consistent with the findings of a previous study using the same dose [16].
IR is the outcome of body weight, FM, inflammation and oxidative stress. To explore the relationship between changes in serum histidine and IR and its risk factors, we analysed the data using correlation analyses in the overall population. Changes in serum histidine were correlated with the changes in HOMA-IR, NEFA, TNF-α, SOD, GSH-Px, WC, FM and BMI, and were still significant after further adjustment for age and serum histidine, protein intake, physical activity, alcohol use, current smoking and menopause at baseline in the partial correlation analyses. Thus, improved insulin sensitivity and alleviation of inflammation and oxidative stress could be due to the increased serum histidine.
Histidine is a free radical scavenger and can chelate divalent metal ions [23, 24]. Its effects against oxidative stress have been well investigated in animals and cells. Histidine has beneficial effects on liver and lung injury in rats and has been reported to protect against diabetic complications in a mouse model of diabetes through its actions against oxidative stress [25–27]. It can restrict accumulation of free radicals and delay activation of extracellular signal-regulated kinase and c-jun N terminal kinase in neuronal cells [28].
Although the mechanisms of the effects of histidine against oxidative stress are apparent, it is still unclear how histidine improves inflammation in obesity. To answer this question, we conducted an in vitro study in human adipocytes. After incubation of adipocytes with PA for 24 h to mimic the inflammatory status of obesity or MetS, the critical point (pAkt) of the insulin-signalling pathway was significantly inhibited; IL6 and TNF mRNA expression and NF-κB protein production were significantly increased. Subsequent incubation with 0, 1, 5 and 10 mmol/l histidine for 24 h, showed that histidine could reverse the effect of PA on the key protein pAkt and decrease levels of pro-inflammatory cytokines and NF-κB in a dose-dependent manner. Additionally, the effects of PA were significantly decreased, and histidine could not influence the pAkt and pro-inflammatory cytokine levels in adipocytes treated with NF-κB inhibitor. Taken together, our in vitro study demonstrated that histidine could improve IR and suppress pro-inflammatory cytokine expression probably through the NF-κB pathway in adipocytes. Likewise, studies have demonstrated that histidine could reduce the levels of IL-6, TNF-α and CRP in animal models of liver and lung injury, diabetes or acute inflammation [25–27, 29]. Some in vitro studies also reported that histidine inhibited hydrogen peroxide- and TNF-α-induced IL-8 secretion at the transcriptional level in intestinal epithelial cells [30] and IL-6 production in human coronary arterial endothelial cells [31]. Our study further confirms the anti-inflammatory effects of histidine in obese human and human adipocytes.
The increased adiponectin observed in our study might be explained by the actions of histidine against inflammation and oxidative stress. As TNF-α suppresses the transcription of adiponectin in adipocytes [32], histidine may increase adipocytes’ secretion of adiponectin by suppressing inflammation and oxidative stress. In addition, inflammation and oxidative stress could stimulate stearolysis and apoptosis of adipocytes, resulting in increased NEFA, while adiponectin could increase the clearance of NEFA from the circulation by increasing skeletal uptake and oxidation of lipid [33]; this may explain why NEFA levels decreased after histidine supplementation.
We also observed significantly reduced BMI and FM in the histidine supplementation group. A recent animal study provides a clue that histidine significantly lowers body weight and FM through diminishing the activity and mRNA expression of fatty acid synthase (FAS), sterol regulatory element-binding proteins-1 (SREBF1) and the gene for malic enzyme in high-fat-diet-induced hepatic steatosis [34]. The close links between obesity, inflammation, oxidative stress, reduced BMI and FM might also play a role in the improvement of IR.
Amino acids are not only common nutrients but are also cell-signalling molecules, regulators of gene expression and the protein phosphorylation cascade [35]. Our findings suggest that obese individuals with MetS or IR might benefit from foods rich in histidine or histidine supplementation, without side effects, in multiple ways. Thus, the underlying mechanisms of histidine’s effects need to be further investigated and more attention should be paid to the metabolism of specific amino acids in obese individuals.
This study has certain limitations. Because previous studies found that women were more sensitive to dietary histidine and energy intake than men [14, 36], we chose women to participate in our study. Therefore, the findings may not be generalised to the total population.
In conclusion, this randomised, double-blind, placebo-controlled trial found that histidine could improve IR, low-grade inflammation and oxidative stress; it could also reduce BMI, FM and NEFA in obese women with MetS. Additionally, we found that histidine could improve IR and suppress pro-inflammatory cytokine expression in adipocytes, possibly through the NF-κB pathway.
Abbreviations
- 2 h-PG:
-
2 h postload glucose
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- ANCOVA:
-
Analysis of covariance
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- Crea:
-
Serum creatinine
- CRP:
-
C-reactive protein
- DBP:
-
Diastolic blood pressure
- FBG:
-
Fasting blood glucose
- FM:
-
Fat mass
- GGT:
-
γ-glutamyl transferase
- GSH-Px:
-
Glutathione peroxidase
- IR:
-
Insulin resistance
- MetS:
-
Metabolic syndrome
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa-B
- OA:
-
Oleic acid
- PA:
-
Palmitic acid
- QNZ:
-
Quinazoline
- TC:
-
Total cholesterol
- TG:
-
Triacylglycerol
- SBP:
-
Systolic blood pressure
- SOD:
-
Superoxide dismutase
- UA:
-
Serum uric acid
- WC:
-
Waist circumference
References
Reaven GM (2011) Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: similarities and differences. J Clin Hypertens 13:238–243
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871
Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM (2012) Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia 55:625–631
Bloomgarden ZT (2011) World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease: part 2. Diabetes Care 34:e126–e131
Henriksen EJ, Diamond-Stanic MK, Marchionne EM (2011) Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 51:993–999
Schipper HS, Nuboer R, Prop S et al (2012) Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14++ monocytes. Diabetologia 55:2800–2810
Selvaraju V, Joshi M, Suresh S, Sanchez JA, Maulik N, Maulik G (2012) Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease—an overview. Toxicol Mech Methods 22:330–335
Watanabe M, Suliman ME, Qureshi AR et al (2008) Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. Am J Clin Nutr 87:1860–1866
Niu YC, Feng RN, Hou Y et al (2012) Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br J Nutr 108:57–61
Mihalik SJ, Michaliszyn SF, de las Heras J et al (2012) Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care 35:605–611
Zhou BF (2002) Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 15:83–96
Alberti KG, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062
Rabbani GH, Sack DA, Ahmed S et al (2005) Antidiarrheal effects of l-histidine-supplemented rice-based oral rehydration solution in the treatment of male adults with severe cholera in Bangladesh: a double-blind, randomized trial. J Infect Dis 191:1507–1514
Okubo H, Sasaki S (2005) Histidine intake may negatively correlate with energy intake in human: a cross-sectional study in Japanese female students aged 18 years. J Nutr Sci Vitaminol (Tokyo) 51:329–334
Zlotkin SH (1989) Nutrient interactions with total parenteral nutrition: effect of histidine and cysteine intake on urinary zinc excretion. J Pediatr 114:859–864
Schechter PJ, Prakash NJ (1979) Failure of oral l-histidine to influence appetite or affect zinc metabolism in man: a double-blind study. Am J Clin Nutr 32:1011–1014
Li Y, Wang C, Zhu K, Feng RN, Sun CH (2010) Effects of multivitamin and mineral supplementation on adiposity, energy expenditure and lipid profiles in obese Chinese women. Int J Obes 34:1070–1077
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
He Y, Zhang H, Teng J, Huang L, Li Y, Sun C (2011) Involvement of calcium-sensing receptor in inhibition of lipolysis through intracellular cAMP and calcium pathways in human adipocytes. Biochem Biophys Res Commun 404:393–399
Fears CYGJ, Stewart JE Jr, Annis DS, Mosher DF, Bornstein P, Gladson CL (2005) Low-density lipoprotein receptor-related protein contributes to the antiangiogenic activity of thrombospondin-2 in a murine glioma model. Cancer Res 65:9338–9346
Choi S, Kim JH, Roh EJ, Ko MJ, Jung JE, Kim HJ (2006) Nuclear factor-kappaB activated by capacitative Ca2+ entry enhances muscarinic receptor-mediated soluble amyloid precursor protein (sAPPalpha) release in SH-SY5Y cells. J Biol Chem 281:12722–12728
Geliebter AA, Hashim SA, Van Itallie TB (1981) Oral l-histidine fails to reduce taste and smell acuity but induces anorexia and urinary zinc excretion. Am J Clin Nutr 34:119–120
Babizhayev MA, Seguin MC, Gueyne J, Evstigneeva RP, Ageyeva EA, Zheltukhina GA (1994) l-carnosine (beta-alanyl-l-histidine) and carcinine (beta-alanylhistamine) act as natural antioxidants with hydroxyl-radical-scavenging and lipid-peroxidase activities. Biochem J 304(Pt 2):509–516
Lee JW, Miyawaki H, Bobst EV, Hester JD, Ashraf M, Bobst AM (1999) Improved functional recovery of ischemic rat hearts due to singlet oxygen scavengers histidine and carnosine. J Mol Cell Cardiol 31:113–121
Yan SL, Wu ST, Yin MC, Chen HT, Chen HC (2009) Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci 74:H259–H265
Cuzzocrea S, Genovese T, Failla M et al (2007) Protective effect of orally administered carnosine on bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 292:L1095–L1104
Lee YT, Hsu CC, Lin MH, Liu KS, Yin MC (2005) Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol 513:145–150
Kulebyakin K, Karpova L, Lakonsteva E, Krasavin M, Boldyrev A (2012) Carnosine protects neurons against oxidative stress and modulates the time profile of MAPK cascade signaling. Amino acids 43:91–96
Farshid AA, Tamaddonfard E, Yahyaee F (2010) Effects of histidine and N-acetylcysteine on diclofenac-induced anti-inflammatory response in acute inflammation in rats. Indian J Exp Biol 48:1136–1142
Son DO, Satsu H, Shimizu M (2005) Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett 579:4671–4677
Hasegawa S, Ichiyama T, Sonaka I et al (2012) Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin Exp Immunol 167:269–274
Tilg H, Moschen AR (2008) Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci (Lond) 114:275–288
Bernstein EL, Koutkia P, Ljungquist K, Breu J, Canavan B, Grinspoon S (2004) Acute regulation of adiponectin by free fatty acids. Metabolism 53:790–793
Mong MC, Chao CY, Yin MC (2011) Histidine and carnosine alleviated hepatic steatosis in mice consumed high saturated fat diet. Eur J Pharmacol 653:82–88
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Kasaoka S, Kawahara Y, Inoue S et al (2005) Gender effects in dietary histidine-induced anorexia. Nutrition 21:855–858
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
YL and CHS were responsible for the study concept and design. RNF and XWS conducted the experiment and collected data. YCN, QL, CW, FCG and CZ were responsible for analysis and interpretation of data. RNF, YCN and QL drafted the manuscript. All authors revised the manuscript critically for intellectual content and approved the final version to be published.
Author information
Authors and Affiliations
Corresponding authors
Additional information
R. N. Feng and Y. C. Niu are joint first authors of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 7 kb)
ESM Table 1
(PDF 28 kb)
ESM Table 2
(PDF 29 kb)
ESM Table 3
(PDF 29 kb)
Rights and permissions
About this article
Cite this article
Feng, R.N., Niu, Y.C., Sun, X.W. et al. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: a randomised controlled trial. Diabetologia 56, 985–994 (2013). https://doi.org/10.1007/s00125-013-2839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2839-7