Abstract
Aims/hypothesis
The aim of this work was to compare the glucose-lowering efficacy of dipeptidyl peptidase-4 (DPP-4) inhibitors between Asian and non-Asian patients with type 2 diabetes.
Methods
We searched MEDLINE, EMBASE, LILACS, CENTRAL, ClinicalTrials.gov and conference proceedings. Studies were eligible if they were randomised controlled trials with a treatment duration of at least 12 weeks, compared a DPP-4 inhibitor with a placebo as either monotherapy or oral combination therapy, had information on ethnicity and HbA1c values and were published or described in English. A systematic review and meta-analysis with a meta-regression analysis was conducted.
Results
Among 809 potentially relevant studies, 55 trials were included. A meta-analysis revealed that DPP-4 inhibitors lowered HbA1c to a greater extent in studies with ≥50% Asian participants (weighted mean difference [WMD] −0.92%; 95% CI −1.03, −0.82) than in studies with <50% Asian participants (WMD −0.65%; 95% CI −0.69, −0.60). The between-group difference was −0.26% (95% CI −0.36, −0.17, p < 0.001). The baseline BMI significantly correlated with the HbA1c-lowering efficacy of DPP-4 inhibitors. The RR of achieving the goal of HbA1c <7.0% (53.0 mmol/mol) was higher in studies with ≥50% Asian participants (3.4 [95% CI 2.6, 4.7] vs 1.9 [95% CI 1.8, 2.0]). The fasting plasma glucose-lowering efficacy was higher with monotherapy in the Asian-dominant studies, but the postprandial glucose-lowering efficacy and changes in body weight were comparable between the two groups.
Conclusions/interpretation
DPP-4 inhibitors exhibit a better glucose-lowering efficacy in Asians than in other ethnic groups; this requires further investigation to understand the underlying mechanism, particularly in relation to BMI.
Similar content being viewed by others
Introduction
The pathophysiology of type 2 diabetes includes both insulin resistance and decreased insulin secretion. The contribution of these components is within a spectrum ranging from predominant insulin resistance to a predominant insulin secretory defect [1]. There is a growing body of evidence that the pathophysiology of type 2 diabetes differs by ethnic group [2–4]. Comparing Asian and white patients with type 2 diabetes, Asian patients are characterised by a relatively lower BMI [4], higher amounts of visceral fat with a given BMI or waist circumference [5, 6] and a predominant insulin secretory defect [7–9]. In patients with type 2 diabetes, the insulin secretory defect is more prominent in Asian than in white individuals [7, 10, 11]. The reasons for decreased insulin secretion in Asian patients with type 2 diabetes are yet to be determined but may be explained by lower beta cell mass, impaired beta cell function and genetic differences [2–4].
Incretin hormones are secreted after a meal to increase glucose-dependent insulin secretion [12, 13]. The incretin effect explains approximately 50–70% of insulin secretion after an oral glucose load in healthy individuals but secretion is markedly reduced (approximately 10–30%) in patients with type 2 diabetes [14–16]. To date, two incretin hormones have been identified: glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) [12, 13]. GLP-1 and GIP are readily degraded into inactive metabolites by dipeptidyl peptidase-4 (DPP-4), resulting in very short half-lives in the circulation [12, 13]. The clinical use of DPP-4 inhibitors, which increase the concentration of the active forms of GLP-1 and GIP, is becoming increasingly popular.
Given the contribution of the insulin secretory defect and insulin resistance to differences in the pathophysiology of type 2 diabetes between Asians and non-Asians, the glucose-lowering efficacy of DPP-4 inhibitors should differ by ethnic group. Thus, we performed a systematic review and meta-analysis on the glucose-lowering efficacy of DPP-4 inhibitors in Asians and non-Asians and conducted meta-regression analyses to identify factors contributing to the difference between the two groups.
Methods
The conduct and results of the study are reported in accordance with the PRISMA statement [17].
Data sources and searches
A systematic search was performed to identify potentially relevant studies in the MEDLINE, EMBASE, CENTRAL, LILACS and ClinicalTrials.gov databases up to February 2012. In addition, the electronically searchable abstracts from the scientific conferences of the ADA from 2004 to 2011 and the EASD from 2008 to 2011 were examined. Search terms used in this study are presented in electronic supplementary material (ESM) Methods.
Eligibility criteria and study selection
Studies were eligible for inclusion if they met the following criteria: (1) randomised controlled trials comparing a DPP-4 inhibitor with a placebo as either monotherapy or combination therapy with other oral glucose-lowering drugs in patients with type 2 diabetes; (2) a treatment duration of at least 12 weeks; (3) information on the ethnicity of the study participants; (4) information on HbA1c values and (5) studies published or described in English. Eligibility was assessed independently by two authors (Y. G. Kim and T. J. Oh) and any disagreements were resolved by consensus. Studies were excluded if the change in the HbA1c value from the baseline to the end of follow-up in each treatment and control group was unavailable. Duplicate studies and extended studies from original studies were excluded. Studies performed in individuals with renal or hepatic dysfunction were excluded.
Data extraction
Data extraction was independently conducted by two authors (Y. G. Kim and T. J. Oh). Any disagreements were resolved by consensus with other authors (S. Hahn and Y. M. Cho). The following data were extracted from the eligible studies: name of the first author, year of publication, country where the study was conducted, treatment regimen, number of participants, duration of diabetes, age, percentage of Asians, percentage of men, baseline HbA1c values and baseline BMI.
For dose-range studies, only data arising from currently approved doses were extracted. In the absence of such data, the data for equivalent amounts of daily doses were used. If no approved dose was used, data for a dose having a maximal HbA1c-lowering efficacy were extracted. If a study had two or three comparisons (one monotherapy arm and one or two combination therapy arms) [18, 19], each comparison was treated separately. When there was a comparison for different administration times in a day (morning vs evening) [20], the data for a morning dose were used. Some data not available in the original papers were extracted supplementarily using information from a full report available at a trial registry, ClinicalTrials.gov [18, 19, 21–61]. With regard to the ethnicity information, we followed the classification of ‘Asian’ used by the authors of each study. If a study did not disclose ethnic information but was conducted in Asian countries with a relatively homogeneous population (e.g. Korea, China, Japan and Taiwan), we assumed the study participants were Asian.
Study quality and risk of bias assessment
We used the Cochrane Collaboration’s tool [62] to assess the risk of bias in adequacy of sequence generation, allocation concealment, type of blinding of participants and personnel, incomplete outcome data, selective outcome reporting and other biases. A detailed description is available in ESM Methods. To assess publication bias, we used a funnel plot and Egger’s test.
Data synthesis and analysis
The primary outcome of this meta-analysis was the HbA1c-lowering efficacy of DPP-4 inhibitors at the end of the study. Secondary outcomes were the effect of DPP-4 inhibitors on fasting plasma glucose (FPG), 2 h postprandial glucose (PPG) following a 75 g glucose load or a standard meal, body weight and achievement of the HbA1c <7.0% (53.0 mmol/mol) goal in the randomised patients. In each study, for outcomes measured on a continuous scale, a weighted mean difference (WMD) in the mean change of the outcome from the baseline to the end of the study between the treatment and the comparison groups was calculated with a 95% CI. An RR was calculated with a 95% CI for a dichotomised outcome. The pooled effect was estimated using a random-effects model. The I 2 statistic and the χ 2 test were used to evaluate the statistical heterogeneity. We conducted meta-regression analyses to test the subgroup differences between ethnic groups and to investigate the sources of the differences by considering some pre-specified covariates: age, percentage of men, percentage of Asian persons, BMI, duration of treatment, duration of diabetes and baseline HbA1c level. When a correlation coefficient was considered between particular variables, the coefficient was derived from the determinant coefficient obtained from the corresponding meta-regression analysis. According to the treatment regimen, the eligible studies were categorised into a ‘monotherapy’ or an ‘oral combination therapy’ group. If the percentage of Asian participants was larger than or equal to 50% in a study, we categorised the study as an Asian-dominant study. Studies were additionally divided into high-BMI groups (BMI ≥30 kg/m2) and low-BMI groups (BMI <30 kg/m2). All analyses were performed using the Stata statistical package (version 12; Stata Corp, College Station, TX, USA).
Results
Search results and study characteristics
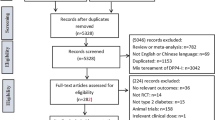
Of the 702 references identified from the four databases, 383 duplicates were eliminated. Of the remaining 319 potentially relevant articles, 259 were excluded after reviewing the titles and abstracts. The full texts of 60 articles were reviewed, and 51 articles were included in the analysis. Of the 107 potentially relevant clinical trials additionally identified from ClinicalTrials.gov (www.clinicaltrials.gov, accessed 8 February 2012), four studies were included in the analysis. Detailed reasons for exclusion are shown in Fig. 1. There were no eligible studies in the ADA or EASD abstracts. We retrieved a total of 55 studies reporting 58 comparison pairs that met the selection criteria for a total of 18,328 study participants (10,270 randomised to treatment group and 8,058 randomised to the comparison group). The doses of DPP-4 inhibitors analysed in this study and a summary of the included studies are shown in Table 1.
Quality of included studies and publication bias assessments
Fifty-four of the 55 included studies achieved a double blindness for the participants and the personnel. For the incomplete outcome data, 49 studies were categorised as low risk, one as unclear and five as high risk. The sequence generation was unclear in 30 studies, and allocation concealment was unclear in approximately 80% of the studies. There was no particular indication for selective reporting from any included studies and 88% (51 of 58) were at low risk for other biases (ESM Table 1, ESM Fig. 1). The Egger’s test and a funnel plot suggested that there was no asymmetric pattern, and no particular concern regarding a publication bias was given to the analyses in the current study (ESM Fig. 2).
Primary outcome: HbA1c-lowering efficacy
A pooling of HbA1c data was performed for 57 pairs from 54 of the 55 studies. One study [37] was excluded because the SD was not available in an adequate form. The WMDs in the changes of HbA1c from the baseline value are depicted in ESM Fig. 3. Overall, the difference between the treatment group and comparison group was −0.72% (95% CI −0.77, −0.67; I 2 = 67.6%) (Fig. 2a). There was no difference in HbA1c-lowering efficacy among different DPP-4 inhibitors (data not shown). We conducted a subgroup analysis by the percentage of Asian participants. The median (range) duration of diabetes was 6.3 (2.0–13.7) and 4.5 (1.4–9.9) years in the Asian-dominant and non-Asian-dominant studies, respectively. The median (range) baseline HbA1c was 7.9 (7.4–9.7)% (62.8 [57.4–82.5] mmol/mol) and 8.3 (7.7–9.9)% (67.2 [60.7–84.7] mmol/mol) in the Asian-dominant and non-Asian-dominant studies, respectively. In studies with <50% Asian participants (non-Asian-dominant studies, n = 41), HbA1c changed by −0.65% (95% CI −0.69, −0.60; I 2 = 35.4%), while in studies with ≥50% Asian participants (Asian-dominant studies, n = 13), HbA1c changed by −0. 92% (95% CI −1.03, −0.82; I 2 = 74.8%). The difference between the two groups was −0.26% (95% CI −0.36, −0.17), which was statistically significant (p < 0.001), suggesting that the HbA1c-lowering efficacy of DPP-4 inhibitors was higher in the Asian-dominant studies than in the non-Asian-dominant studies.
In the monotherapy trials, the overall difference in HbA1c change from baseline between the treatment group and comparison group was −0.74% (95% CI −0.84, −0.64; I 2 = 73.9%) (Fig. 2a). HbA1c changed by −0.64% (95% CI −0.70, −0.57; I 2 = 13.8%) in the non-Asian-dominant studies whereas it changed by −1.01% (95% CI −1.14, −0.88; I 2 = 58.3%) in the Asian-dominant studies. The difference between the two groups was −0.38% (95% CI −0.52, −0.23), which was statistically significant (p < 0.001). In trials with oral combination therapy, the overall difference of HbA1c change from the baseline between treatment group and comparison group was −0.70% (95% CI −0.76, −0.65; I 2 = 61.2%). HbA1c changed by −0.66% (95% CI −0.71, −0.60; I 2 = 44.3%) in the non-Asian-dominant studies whereas it changed by −0.85% (95% CI −0.97, −0.72; I 2 = 72.3%) in the Asian-dominant studies. The difference between the two groups was −0.18% (95% CI −0.31, −0.05), which was statistically significant (p = 0.006). The HbA1c-lowering efficacy exhibited no difference whether a DPP-4 inhibitor was added on top of metformin (WMD −0.68% [95% CI −0.77, −0.60]; I 2 = 61.0%) or sulfonylurea (WMD −0.68% [95% CI −0.90, −0.47]; I 2 = 74.6%).
The univariate meta-regression analyses (Fig. 3a, c; ESM Fig. 4) revealed that the percentage of Asian participants (p < 0.001), BMI (p < 0.001), age (p = 0.006), percentage of men (p = 0.027), duration of disease (p = 0.005) and duration of treatment (p = 0.013) were significantly correlated with the change in HbA1c from baseline. Interestingly, the percentage of Asian participants was negatively correlated with average BMI (r = −0.95) (Fig. 3b). As the percentage of Asian participants increased or the BMI decreased, the change in HbA1c became larger. The BMI distribution of the Asian-dominant studies ranged from 23.8 to 28.4 kg/m2 and that of the non-Asian-dominant studies ranged from 28.3 to 33.3 kg/m2. Therefore, BMI could be a confounding factor in the apparent relationship between the percentage of Asian participants and the HbA1c-lowering efficacy of the DPP-4 inhibitors. The percentage of Asian participants was independently associated with the change in HbA1c from baseline, even in a multiple meta-regression analysis adjusted for treatment duration, percentage of men, duration of diabetes, age and baseline HbA1c level (ESM Table 2). However, the relationship between the percentage of Asians and the change in HbA1c from baseline was no longer significant after adjusting for BMI (p = 0.491, ESM Table 2), which may be the result of the statistical multicollinearity. The correlation between BMI and HbA1c-lowering efficacy was dependent upon the study populations. In Asian-dominant studies there was a clear correlation between BMI and the HbA1c-lowering effect, but in non-Asian-dominant studies there was no such correlation (ESM Table 2). Similarly, there was no correlation between BMI and the HbA1c-lowering efficacy of the DPP-4 inhibitors in the studies in which the average BMI was ≥30 kg/m2, but BMI was significantly correlated with the HbA1c-lowering efficacy of the DPP-4 inhibitors in the studies in which the average BMI was <30 kg/m2 (Fig. 3d).
Correlations between HbA1c-lowering efficacy and percentage of Asian participants or BMI. (a) Correlation between HbA1c-lowering efficacy and percentage of Asian participants (p < 0.001). (b) Correlation between BMI and percentage of Asian participants (r = 0.95, p < 0.001). (c) Correlation between HbA1c-lowering efficacy and BMI (p < 0.001). (d) HbA1c-lowering efficacy in lower- and higher-BMI groups. The equation for the trend line on the left is Y = 0.072 × X − 2.7 (p < 0.001) and the equation for the trend line on the right is Y = −0.016 × X − 0.2 (p = 0.623), where ‘p’ represents p values of regression coefficients and ‘r’ represents correlation coefficient calculated from a meta-regression analysis
In addition to the meta-regression analysis, to further resolve the issues associated with heterogeneity and the arbitrary cutoff of the proportion of Asians for Asian-dominance, we classified the included studies into three groups according to the proportion of the Asians participants: low Asian zone (Asian ≤20%), intermediate Asian zone (<20% Asian ≤80%) and high Asian zone (Asian >80%) (ESM Table 3). Consequently, we could reduce the heterogeneity of three zones compared with that of the binary classification (Asian-dominant vs non-Asian-dominant). The difference in HbA1c-lowering efficacy between the high Asian zone and the low Asian zone was −0.29% (95% CI −0.39, −0.19), which was statistically significant (p < 0.001).
Secondary outcome: FPG
The changes in FPG from baseline are shown in Fig. 2b. We analysed 52 pairs from 49 studies involving FPG. Overall, FPG changed from baseline by −1.08 mmol/l (95% CI −1.18, −0.98; I 2 = 52.1%). The change in FPG from baseline was −1.03 mmol/l (95% CI −1.14, −0.92; I 2 = 40.3%) in the non-Asian-dominant studies and −1.23 mmol/l (95% CI −1.47, −1.00; I 2 = 70.9%) in the Asian-dominant studies. In the monotherapy trials, the overall decrease in FPG was significantly larger in the Asian-dominant studies than in non-Asian-dominant studies; the difference between the two groups was −0.45 mmol/l (95% CI −0.79, −0.10). However, in oral combination therapy trials, there was no difference in the change in FPG from baseline between the two groups.
Secondary outcome: 2 h PPG
The changes in 2 h PPG from baseline are shown in Fig. 2c. We analysed 25 pairs from 22 studies involving PPG. Overall 2 h PPG change from baseline was −2.60 mmol/l (95% CI −2.83, −2.37; I 2 = 38.1%). The change was −2.86 mmol/l (95% CI −3.36, −2.36; I 2 = 65.6%) in the Asian-dominant studies and −2.45 mmol/l (95% CI −2.68, −2.22; I 2 = 0.0%) in the non-Asian-dominant studies. Although the mean value of the decrease in 2 h PPG was numerically larger in the Asian-dominant studies, the difference was not statistically significant. A similar trend was observed in both the monotherapy and oral combination therapy trials.
Secondary outcome: RR for achieving HbA1c <7.0% (53.0 mmol/mol)
Thirty-one pairs from 28 studies were used to estimate the RR for achieving HbA1c <7.0% (53.0 mmol/mol). The RR was significantly larger in the Asian-dominant studies than in the non-Asian-dominant studies (3.4 [95% CI 2.6, 4.7] vs 1.9 [95% CI 1.8, 2.0]) (ESM Fig. 5).
Secondary outcome: body weight
Thirty pairs from 29 studies were used for the analysis on body weight. Overall body weight change from baseline was 0.52 kg (95% CI 0.37, 0.67; I 2 = 47.4%). There was no significant difference in body weight change between the Asian-dominant studies and the non-Asian-dominant studies (ESM Fig. 6).
Discussion
In this systematic review and meta-analysis, the HbA1c-lowering efficacy of DPP-4 inhibitors in type 2 diabetes was higher in Asians than in other ethnic groups. Differences in BMI across ethnic groups may mediate the HbA1c-lowering efficacy of DPP-4 inhibitors. In the current meta-analysis, there was no correlation between the BMI and the HbA1c-lowering efficacy of DPP-4 inhibitors in the studies in which the average BMI was ≥30 kg/m2, but BMI was significantly correlated with the HbA1c-lowering efficacy of DPP-4 inhibitors in the studies in which the average BMI was <30 kg/m2. There was no difference in body weight change from baseline between the Asian- and the non-Asian-dominant studies, which suggests that the baseline BMI might influence the glucose-lowering effect of DPP-4 inhibitors. In fact, a Japanese study revealed a significant correlation (r = 0.419, p = 0.0023) between baseline BMI and HbA1c levels after 16 weeks of sitagliptin treatment in patients with type 2 diabetes and a BMI of 24.1 ± 5.0 kg/m2 [63], which suggests that a lower BMI is a predictor of a good response to a DPP-4 inhibitor. Collectively, the different BMIs among ethnic groups may contribute to the differences in the glucose-lowering response to DPP-4 inhibitors.
Because BMI is highly correlated with insulin sensitivity [64], the apparent effect of BMI on the HbA1c-lowering efficacy observed in our study may be mediated by differences in insulin sensitivity among different ethnic groups. However, factors other than BMI may influence the insulin sensitivity of different ethnic groups. In a study examining 531 first-degree relatives of individuals with type 2 diabetes in the USA [65], Asian–Americans with a normal glucose tolerance or impaired glucose regulation were more insulin-sensitive than all other ethnic groups, including African–Americans, Hispanic–Americans and persons of European descent. These findings remained significant even after adjusting for the effect of BMI. Although clinical variables reflecting insulin sensitivity, such as direct or indirect indices for insulin sensitivity or amount of visceral fat, were not available in this analysis, those variables could provide a missing link between ethnicity and treatment response to DPP-4 inhibitors.
If the pharmacokinetic properties of DPP-4 inhibitors differ between Asians and non-Asians largely because of differences in body size, the glucose-lowering efficacy of DPP-4 inhibitors may differ by ethnic group. However, the clinical pharmacological characteristics of several DPP-4 inhibitors are similar in different ethnic groups [66, 67]. The pharmacokinetic variables of sitagliptin are reported not to be different among whites, blacks, Hispanics and Asians [66]. In a study involving 60 healthy Chinese participants, vildagliptin had pharmacokinetic variables that were similar to non-Asians [67]. Therefore, we cannot explain the better glucose-lowering efficacy of DPP-4 inhibitors in Asians by different pharmacokinetic properties.
According to the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia (DECODA) [68] and the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) [69] studies, the prevalence of post-challenge hyperglycaemia is higher in Asian than in white persons. In the DECODA study, more than half the patients with diabetes had isolated postprandial hyperglycaemia [70], which is additionally a powerful predictor of cardiovascular disease and premature death [71]. DPP-4 inhibitors are known to reduce both FPG and PPG [72]. In particular, DPP-4 inhibitors effectively lower PPG by increasing active GLP-1 and decreasing glucagon levels [73]. We examined whether the efficacy of DPP-4 inhibitors in lowering FPG or PPG differs between Asian- and non-Asian-dominant studies. The FPG-lowering efficacy with DPP-4 inhibitor monotherapy was higher in the Asian-dominant studies than in the non-Asian-dominant studies, while the PPG-lowering efficacy was not significantly different between two groups. Because the number of studies reporting FPG or PPG from baseline was relatively smaller and the PPG values were measured with either a 75 g glucose load or a standardised meal, further studies are needed to examine the difference in FPG- or PPG-lowering efficacy of DPP-4 inhibitors among different ethnic groups.
There was no effect of baseline HbA1c on the efficacy of DPP-4 inhibitors in our analysis. However, Deacon suggested that a higher baseline HbA1c would be a predictor of a greater HbA1c reduction with a DPP-4 inhibitor [72]. Approximately 70% of the studies reviewed in Deacon’s paper were also included in our study. However, the analytical methods were different. The values presented by Deacon were delta HbA1c simply in the DPP-4 inhibitor group rather than the change in the delta HbA1c between the placebo and the DPP-4 inhibitor group. If we plot our data in the same way as Deacon, the graph looks similar (data not shown). Therefore, at least in our analysis, the contribution of BMI or Asian proportion outweighs the effect of baseline HbA1c in determining the placebo-subtracted HbA1c-lowering effect.
There are some limitations to this study. First, we did not separate the studies including South Asians (e.g. Indians) from the studies mainly comprised of East Asians. Asian populations are ethnically heterogeneous and have different demographic, cultural and socioeconomic characteristics. Insulin resistance may be the major contributor to the pathogenesis of type 2 diabetes in South Asian populations [3] but not East Asian populations. Second, because the analysis was based only on aggregated information at the study level and was exploratory in nature, additional studies are needed to explain the mechanisms for the different effect by ethnicity. Although DPP-4 inhibitors showed an overall significant treatment effect in a meta-analysis of the current literature, there was substantial heterogeneity in terms of the size of the effect among the studies, whose clinical characteristics additionally differ. To confirm our observation, a patient-level data analysis or a prospective randomised study including different ethnic groups should be performed.
In conclusion, the glucose-lowering efficacy of DPP-4 inhibitors is higher in Asians than other ethnic groups. Different BMIs may contribute to this difference in treatment response to DPP-4 inhibitors. Considering that Asia is the epicentre of the current worldwide epidemic of diabetes [2], this study suggests the need for ethnic-specific guidelines for the pharmacological treatment of diabetes.
Abbreviations
- DECODA:
-
Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia
- DECODE:
-
Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe
- DPP-4:
-
Dipeptidyl peptidase-4
- FPG:
-
Fasting plasma glucose
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP-1:
-
Glucagon-like peptide-1
- PPG:
-
Postprandial glucose
- WMD:
-
Weighted mean difference
References
American Diabetes Association (2012) Diagnosis and classification of diabetes mellitus. Diabetes Care 35(suppl 1):S64–S71
Chan JC, Malik V, Jia W et al (2009) Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301:2129–2140
Ramachandran A, Ma RC, Snehalatha C (2010) Diabetes in Asia. Lancet 375:408–418
Yoon KH, Lee JH, Kim JW et al (2006) Epidemic obesity and type 2 diabetes in Asia. Lancet 368:1681–1688
Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL (2007) Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 86:353–359
Park YW, Allison DB, Heymsfield SB, Gallagher D (2001) Larger amounts of visceral adipose tissue in Asian Americans. Obes Res 9:381–387
Fukushima M, Suzuki H, Seino Y (2004) Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 66(suppl 1):S37–S43
Matsumoto K, Miyake S, Yano M et al (1997) Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care 20:1562–1568
Rattarasarn C, Soonthornpan S, Leelawattana R, Setasuban W (2006) Decreased insulin secretion but not insulin sensitivity in normal glucose tolerant Thai subjects. Diabetes Care 29:742–743
Kim YI, Choi CS, Kim SW et al (1998) Changes in serum true insulin and C-peptide levels during oral glucose tolerance test in Koreans with glucose intolerance. J Korean Diabetes Assoc 22:192–198
Tripathy D, Carlsson M, Almgren P et al (2000) Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 49:975–980
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157
Cho YM, Kieffer TJ (2010) K-cells and glucose-dependent insulinotropic polypeptide in health and disease. Vitam Horm 84:111–150
Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsboll T (2011) Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 96:737–745
Knop FK, Vilsboll T, Hojberg PV et al (2007) Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56:1951–1959
Nauck MA, Homberger E, Siegel EG et al (1986) Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 63:492–498
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE (2007) Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 30:1979–1987
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P (2007) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 9:733–745
Goodman M, Thurston H, Penman J (2009) Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res 41:368–373
Raz I, Chen Y, Wu M et al (2008) Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 24:537–550
Charbonnel B, Karasik A, Liu J, Wu M, Meininger G (2006) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 29:2638–2643
Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE (2006) Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 29:2632–2637
Mohan V, Yang W, Son HY et al (2009) Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract 83:106–116
Taskinen M, Rosenstock J, Tamminen I et al (2011) Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metabol 13:65–74
Forst T, Uhlig-Laske B, Ring A et al (2010) Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled type 2 diabetes. Diabet Med 27:1409–1419
Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA (2011) Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 13:258–67
Gomis R, Espadero RM, Jones R, Woerle HJ, Dugi KA (2011) Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 13:653–661
Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q (2009) Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab 11:1145–1152
Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q (2009) Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract 63:46–55
DeFronzo RA, Hissa MN, Garber AJ et al (2009) The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 32:1649–1655
DeFronzo RA, Fleck PR, Wilson CA, Mekki Q (2008) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 31:2315–2317
Pratley RE, Reusch JEB, Fleck PR, Wilson CA, Mekki Q (2009) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 25:2361–2371
Jadzinsky M, Pfutzner A, Paz-Pacheco E, Xu Z, Allen E, Chen R (2009) Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 11:611–622
Rosenstock J, Guilar-Salinas C, Klein E, Nepal S, List J, Chen R (2009) Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin 25:2401–2411
Owens DR, Swallow R, Dugi KA, Woerle HJ (2011) Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med 28:1352–1361
Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I (2011) Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Res Clin Pract 94:217–224
Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R (2009) Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin 25:2401–2411
Iwamoto Y, Taniguchi T, Nonaka K et al (2010) Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J 57:383–394
Nonaka K, Kakikawa T, Sato A et al (2008) Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 79:291–298
Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H (2006) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 49:2564–2571
Dejager S, Razac S, Foley JE, Schweizer A (2007) Vildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 39:218–223
Pi-Sunyer FX, Schweizer A, Mills D, Dejager S (2007) Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract 76:132–138
Pratley RE, Kipnes MS, Fleck PR et al (2009) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metabol 11:167–176
Rosenstock J, Inzucchi SE, Seufert J, Fleck PR, Wilson CA, Mekki Q (2010) Initial combination therapy with alogliptin and pioglitazone in drug-naive patients with type 2 diabetes. Diabetes Care 33:2406–2408
Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K (2011) Alogliptin plus voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension. Curr Med Res Opin 27:21–29
Terra SG, Somayaji V, Schwartz S et al (2011) A dose-ranging study of the DPP-IV inhibitor PF-734200 added to metformin in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes 119:401–407
Hollander P, Li J, Allen E, Chen R (2009) Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab 94:4810–4819
Scott R, Loeys T, Davies MJ, Engel SS (2008) Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 10:959–969
Yoon KH, Shockey GR, Teng R et al (2011) Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and pioglitazone on glycemic control and measures of beta-cell function in patients with type 2 diabetes. Int J Clin Pract 65:154–164
Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P (2006) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 28:1556–1568
Reasner C, Olansky L, Seck TL et al (2011) The effect of initial therapy with the fixed-dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Obes Metab 13:644–652
Kashiwagi A, Kadowaki T, Tajima N et al (2011) Sitagliptin added to treatment with ongoing pioglitazone for up to 52 weeks improves glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig 2:381–390
Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ (2007) Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 30:890–895
Rosenstock J, Kim SW, Baron MA et al (2007) Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 9:175–185
Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S (2007) Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 9:166–174
Kikuchi M, Haneda M, Koya D et al (2010) Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 89:216–223
Bosi E, Dotta F, Jia Y, Goodman M (2009) Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab 11:506–515
Garber AJ, Foley JE, Banerji MA et al (2008) Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab 10:1047–1056
Vilsboll T, Rosenstock J, Yki-Jarvinen H et al (2010) Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 12:167–177
Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S (2007) Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 50:1148–1155
Higgins JPT, Green S (eds) (2005) Cochrane handbook for systematic reviews of interventions 5.1.0. In: The Cochrane Library (Issue 2). Wiley, Chichester
Aso Y, Ozeki N, Terasawa T et al (2012) Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl Res 159:25–31
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE (2002) Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 51:2170–2178
European Medicine Agency (2007) Scientific discussion. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000722/WC500039057.pdf. Accessed 8 February 2012
Hu P, Yin Q, Deckert F et al (2009) Pharmacokinetics and pharmacodynamics of vildagliptin in healthy Chinese volunteers. J Clin Pharmacol 49:39–49
DECODA Study Group; International Diabetes Epidemiology Group (2002) Cardiovascular risk profile assessment in glucose-intolerant Asian individuals—an evaluation of the World Health Organization two-step strategy: the DECODA Study (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia). Diabet Med 19:549–557
DECODE Study Group (1998) Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. BMJ 317:371–375
Qiao Q, Hu G, Tuomilehto J et al (2003) Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 26:1770–1780
Nakagami T (2004) Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 47:385–394
Deacon CF (2011) Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab 13:7–18
DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L (2008) Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 24:2943–2952
Rosenstock J, Sankoh S, List JF (2008) Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab 10:376–386
Hanefeld M, Herman GA, Wu M et al (2007) Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 23:1329–1339
Scott R, Wu M, Sanchez M, Stein P (2007) Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 61:171–180
Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D (2006) Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res 38:423–428
Ristic S, Byiers S, Foley J, Holmes D (2005) Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab 7:692–698
Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K (2011) Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study. Curr Med Res Opin 27:1781–1792
Rhee EJ, Lee WY, Yoon KH et al (2010) A multicenter, randomized, placebo-controlled, double-blind phase II trial evaluating the optimal dose, efficacy and safety of LC 15–0444 in patients with type 2 diabetes. Diabetes Obes Metab 12:1113–1119
Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H (2009) Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 83:233–240
Ahren B, Gomis R, Standl E, Mills D, Schweizer A (2004) Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care 27:2874–2880
Kaku K, Itayasu T, Hiroi S, Hirayama M, Seino Y (2011) Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes Obes Metab 13:1028–1035
Chien MN, Lee CC, Chen WC, Liu SC, Leung CH, Wang CH (2011) Effect of sitagliptin as add-on therapy in elderly type 2 diabetes patients with inadequate glycemic control in Taiwan. Int J Gerontol 5:103–106
Funding
This study received no financial support from any type of foundation or company.
Duality of interest
Y. M. Cho received a lecture fee or consultation fee from MSD, Lilly, Novartis, Astra-Zeneca and Boehringer-Ingelheim.
Contribution statement
YGK, SH and YMC developed the protocol and were responsible for the study design, main concept and statistical analysis. YGK and TJO were responsible for the study selection and data extraction. SH and YMC additionally contributed to data extraction. YGK, SH, SHK, KSP and YMC were contributors to the result interpretation. All authors wrote the initial draft and revised the paper for important intellectual content. All authors were contributors to the final manuscript and have approved the final version.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. G. Kim and S. Hahn contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 148 kb)
ESM Fig. 1
(PDF 86 kb)
ESM Fig. 2
(PDF 136 kb)
ESM Fig. 3
(PDF 179 kb)
ESM Fig. 4
(PDF 196 kb)
ESM Fig. 5
(PDF 180 kb)
ESM Fig. 6
(PDF 153 kb)
ESM Table 1
(PDF 174 kb)
ESM Table 2
(PDF 189 kb)
ESM Table 3
(PDF 190 kb)
Rights and permissions
About this article
Cite this article
Kim, Y.G., Hahn, S., Oh, T.J. et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia 56, 696–708 (2013). https://doi.org/10.1007/s00125-012-2827-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2827-3