Abstract
Aims/hypothesis
Insulin delivery to muscle is rate-limiting for insulin’s metabolic action and is regulated by insulin’s own action to increase skeletal muscle blood flow and to recruit microvasculature. Microvascular dysfunction has been observed in insulin resistant states. We investigated the relation between insulin’s action to recruit microvasculature and its metabolic action in type 1 diabetes.
Methods
Near euglycaemia was obtained by an overnight insulin infusion during 17 inpatient admissions of participants with type 1 diabetes. This was followed by a 2 h 1 mU kg−1 min−1 euglycaemic–hyperinsulinaemic clamp. Microvascular blood volume (MBV) was assessed using contrast-enhanced ultrasound 10 min before and 30 min after starting the clamp.
Results
We observed that, after overnight modest hyperinsulinaemia (average ≈ 286 pmol/l), MBV was positively related to the steady-state insulin sensitivity measured during the subsequent clamp (r = 0.62, p = 0.008). The more marked hyperinsulinaemia during the clamp (average steady-state insulin ≈ 900 pmol/l) increased MBV in the more insulin resistant participants within 30 min but not in the insulin sensitive participants. The change in MBV during the clamp was negatively correlated to the insulin sensitivity (r = −0.55, p = 0.022). As a result, MBV after 30 min of marked hyperinsulinaemia was comparable between the insulin sensitive and resistant participants.
Conclusions/interpretation
We conclude that moderate overnight hyperinsulinaemia recruited microvasculature in the more sensitive participants, while higher levels of plasma insulin were needed for more insulin resistant participants. This suggests that microvascular responsiveness to insulin is one determinant of metabolic insulin sensitivity in type 1 diabetes.
Trial registration:
ClinicalTrials.gov NCT00943787
Funding:
This study is supported by the NIH/NIDDKRO1 DK 51562 grant and the University of Virginia General Clinical Research Center M01 RR 000847 grant.
Similar content being viewed by others
Introduction
In the early 1990s, Baron et al. [1–3] established the concept that, in lean individuals, euglycaemic hyperinsulinaemia at high physiological concentrations stimulates blood flow in skeletal muscle tissue. More recently, it was shown that blood flow distribution stimulated by hyperinsulinaemia mirrored changes in glucose use [4], suggesting that ‘insulin-induced alteration in blood flow patterns could be as important as direct signalling of cells by insulin in establishing the rate of glucose utilisation in vivo’ [5, page 4557]. Insulin thus appears to regulate its metabolic action in part by acting on the vasculature to facilitate its own delivery to skeletal muscle [6], a step known to be rate-limiting for insulin-mediated glucose disposal [7]. It has been recently highlighted that insulin acts on the vasculature not only to dilate resistance vessels (yielding increased blood flow), but also to relax pre-capillary arterioles and recruit microvasculature, thus expanding the surface available for nutrient and insulin delivery [8, 9]. Insulin-mediated capillary recruitment occurs within 10 min, temporally preceding increases in total blood flow [9], and is a key part of insulin action in vivo, accounting for as much as 50% of the insulin-induced increase in glucose uptake [8–11].
Laakso et al. reported that, in insulin resistant obese individuals, leg blood flow was stimulated by pharmacological but not by physiological hyperinsulinaemia [12]. We have reported that metabolic insulin resistance in experimental animals [13] and humans [14] is associated with microvascular insulin resistance within skeletal muscle and that the latter contributes to metabolic insulin resistance [6, 15]. In these latter studies, we used contrast-enhanced ultrasound (CEU) to measure the microvascular blood volume (MBV) in the basal state and during either insulin infusion (euglycaemic clamp) or meal-stimulated insulin secretion [16]. Both obesity [14] and raising plasma non-esterified fatty acid concentrations [17] impaired insulin’s ability to increase MBV and were associated with metabolic insulin resistance.
Most studies of type 1 diabetes have reported moderate degrees of insulin resistance relative to age- and weight-matched controls, even in adolescents with type 1 diabetes [18, 19]. Recent studies have also shown that type 1 diabetic adults and adolescents have impaired flow-mediated dilation in conduit vessels [20, 21]. It is important to understand the effects of insulin on capillary recruitment in type 1 diabetic patients as they exclusively rely on exogenous insulin supplies to ensure glucose disposal and thus maintain safe glucose levels. In the present study we addressed whether a relation between insulin-induced microvascular recruitment and metabolic insulin sensitivity exists in type 1 diabetes, as found in other insulin resistant states.
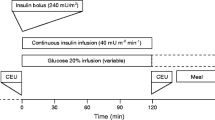
Because individuals with type 1 diabetes are continuously dependent on exogenous insulin, and controlling blood glucose requires peripheral hyperinsulinaemia, we measured MBV after an overnight insulin infusion, which was selected to maintain a participant’s blood sugar between 5.6 and 8.3 mmol/l based upon 30 min glucose measurements and adjustment of the insulin infusion rate. MBV was measured after this overnight, low-dose insulin infusion and participants then received a primed, continuous infusion of insulin for 2 h along with an exogenous glucose infusion to maintain glucose constant at the fasting concentration (euglycaemic–hyperinsulinaemic clamp). Thirty minutes into the euglycaemic clamp, MBV was again measured using CEU imaging techniques. Steady-state insulin sensitivity was determined from the glucose infusion rate required to maintain euglycaemia over the last 30 min of the 2 h insulin clamp. The MBV measurements and insulin-induced change in MBV were related to the metabolic insulin sensitivity.
Methods
Study design and population
Maintenance of euglycaemia requires peripheral hyperinsulinaemia in individuals with type 1 diabetes. If peripheral insulin concentrations in type 1 diabetes are reduced to levels comparable with non-diabetic individuals, hyperglycaemia ensues. In as much as hyperglycaemia per se substantially affects vascular function and responsiveness to insulin, we elected to treat participants with type 1 diabetes overnight with a relatively low-dose insulin infusion to attain near euglycaemia and examine the effects of subsequent marked hyperinsulinaemia using the clamp procedure. Seventeen clamps were performed on 14 patients with type 1 diabetes. For the three patients who were studied twice, the time interval between the clamps was at least 6 months, which allowed us to consider them to be independent. The study was approved by the University of Virginia Internal Review Board and performed at the General Clinical Research Center. All participants gave informed consent.
All participants had an outpatient screening visit that included a history and physical examination, with assessment for orthostatic hypotension. Laboratory testing included a urine microalbumin/creatinine ratio, human chorionic gonadotropin (HCG; females), HbA1c, haematocrit and a comprehensive chemistry panel. The participants were on average 37.2 ± 11.8 years old, had had type 1 diabetes for 19.8 ± 12.2 years and weighed 80.8 ± 12.1 kg. Their mean BMI was 26.3 ± 3.3 kg/m2. All participants were treating their diabetes using an insulin pump or insulin injections, and had HbA1c of 8.0 ± 2.3% (64 ± 25 mmol/mol) measured prior to the clamp admission. We defined a complication index to assess the extent of a patient’s diabetic microvascular complications; this was the number of complications the patient had developed among those related to the kidneys, nerves, feet, eyes and digestive system. The complication index is equal to 0 for no complication and equal to 5 when all of them have developed. The mean complication index was 0.6 ± 1.0. Two participants had nephropathy, as assessed by a ratio of microalbumin to creatinine above 3.4 mg/mmol, and one patient was taking tadalifil, when required, for sexual dysfunction. All participants but one were maintaining regular physical activities. An exhaustive list of the patients’ medications is given in Table 1.
Prior to admission, patients using long or intermediate acting insulin consulted with a study physician for insulin dose adjustment. Long-acting insulin was discontinued 60 h and intermediate acting insulin was discontinued 36 h prior to the clamp procedure. Only soluble or rapid-acting insulin was allowed on the day of the admission. Participants were allowed to take their other outpatient medications, steroids excluded. Patients were asked to try to keep their blood glucose between 5.6 and 8.3 mmol/l and to avoid hypoglycaemia, and to perform frequent finger stick blood glucose measurements (10 per day, at least 30 min apart) for reference values. Participants were admitted to the General Clinical Research Center on the evening prior to study. At 21:30 hours, an intravenous infusion of soluble insulin (Novolin R, Novo Nordisk, Bagsværd, Denmark, 0.1 U/ml saline) was begun and titrated to maintain the participants’ blood glucose overnight between 5.6 and 8.3 mmol/l as measured every 30 min using a YSI analyser (YSI Life Sciences, OH, USA). This infusion was discontinued at 08:30 hours the following morning at the initiation of the clamp procedure. At time 0 an insulin infusion was given via Harvard pump (Harvard Apparatus, MA, USA) as a 20 mU/kg priming over 10 min followed by a constant 1 mU kg−1 min−1 infusion maintained for the next 110 min. Plasma glucose was measured at intervals of 5 min and clamped at basal levels via a variable-rate infusion of 20% dextrose using the equations of DeFronzo et al. [22]. Total plasma insulin concentration was measured by radioimmunoassay (RIA kits, Millipore, MA, USA) at intervals of ≤10 min.
CEU imaging
CEU imaging was performed in the forearm using a SONOS 7500 ultrasound system (Philips Medical Systems, Bothell, WA, USA) and an S3 probe. Pulse inversion imaging was performed at an ultrasound transmit frequency of 1.3 MHz and receive frequency of 3.6 MHz. A 3 ml suspension of octafluoropropane gas-filled lipid microbubbles (Definity, Bristol-Myers Squibb Medical Imaging, North Billerica, MA, USA), diluted in 57 ml saline and continuously infused at a rate of 1.5 ml/min, was used as the contrast medium. CEU imaging was performed 10 min before and 30 min after starting the clamp. Images were obtained at increasing pulsing intervals, from 1 to 20 cardiac cycles, with at least three images acquired at each pulsing interval. This allowed us to quantify increasing microvascular replenishment with microbubbles between the pulses until the beam space was completely refilled. The sequences of ultrasound images were digitalised and stored as TIF files for offline analysis.
Measurement of MBV
When exposed to high-energy ultrasound, the infused microbubbles are destroyed, resulting in a high-amplitude signal. By allowing progressively longer time intervals between pulses, the reappearance of microbubbles within the muscle vasculature results in a time-dependent signal intensity increase. When the space defined by the beam thickness is completely filled, increasing the pulsing interval does not further affect the signal intensity. The replenishment curve, plotted as the mean intensity over a region of interest (ROI) against the pulsing interval in seconds, can be described by an exponential function [23]:
where y is the ROI mean acoustic intensity in decibels (dB) and t the pulsing interval in seconds. The parameter c is the acoustic intensity that would be obtained if the pulsing interval were reduced to 0, that is, the background intensity reflected by the tissues per se. The parameter A represents the plateau of the background-subtracted intensity and β the rate at which the acoustic intensity rises with increasing pulsing intervals. As detailed in [23], MBV was estimated by the parameter A.
ROI selection and quantification of video intensity
To minimise/eliminate the dependence of the ROI selection on the operator and assure consistency in the ROI definition across different sequences, we developed a systematic procedure to select ROI (see [24]). Briefly, the method is based on the normalisation of the intensity of the acoustic signal stored in the image files. The ROI is defined as the union of regions such that the intensity in that region does not exceed a threshold K1 across the frames taken at pulsing interval less than 2 heart beats (this is done to eliminate contributions from bones and large arteries and arterioles to the video intensity) and does not exceed a threshold K2 across all the frames of the sequence (this accounts for the movement of large arteries and arterioles). This method requires having the information for the pulsing interval measured in number of heart beats for each frame. We previously described an algorithm [24] to reconstruct the pulsing interval in number of heart beats from the relative time (in seconds) when relative time is the only information stored in the file. For each sequence, the ROI was selected based on this systematic method and the mean acoustic intensity over the ROI was fitted to the exponential function described by Eq. 1 to obtain estimates of the parameters A, β and c.
Measures
The glucose infusion rate averaged during the steady-state of the euglycaemic–hyperinsulinaemic clamp, denoted as the M value and expressed in μmol kg−1 min−1, provides a measure of whole-body glucose uptake. Whole-body insulin sensitivity (SI), expressed in μmol kg−1 min−1 (pmol/l)−1, was estimated over the final 30 min of the clamp, when a steady-state glucose infusion rate was reached, as the ratio of the M value over the plasma insulin concentration as described by DeFronzo et al. [22]. Because the distribution of SI is skewed to the right, we performed a natural logarithmic transformation on SI and denoted the transformed variable as logSI. Taking the logarithm of the data is a standard statistical transformation aimed at reducing the skewness of the data and increasing the validity of correlation analyses. We defined three measures of plasma insulin concentration as follows: Ip,basal the insulin concentration before the start of the clamp (but after overnight insulin infusion), computed as the mean insulin concentration during the last 30 min preceding the clamp; Ip,priming the insulin concentration reached during the insulin priming, computed as the mean insulin concentration during the first 10 min of the clamp; and Ip,ss the insulin concentration reached during the final 30 min of the clamp; all three measures are expressed in pmol/l. Acquisition of the CEU replenishment curve takes approximately 5 min. Basal MBV (MBVbasal) was assessed 10 min before the start of the clamp and MBV during hyperinsulinaemia was captured at 30 min after starting the clamp (MBV30min). Given the different baseline values, the insulin-induced capillary recruitment was assessed by the relative change in MBV from the basal state to the hyperinsulinaemic state, expressed in percentage:
Statistical analyses
The relationships between logSI and basal MBV and between logSI and ΔMBV were examined using Pearson’s correlation and linear regressions to control for other variables. The participants were divided into two groups using a two-means cluster analysis with respect to standardised logSI and ΔMBV. The standardisation brings all variables to the same order of magnitude by centring the variables on their means and scaling them by their standard deviation. The distance between two points is defined as the Euclidean distance with respect to the standardised logSI and ΔMBV. The K-means analysis creates the groups by minimising the sum, over all groups, of the within-group sums of point-to-group-centroid distances. In other words, participants that are similar in both their logSI and ΔMBV are grouped together. Statistical comparisons between the two groups were then performed using an unpaired t test. For all tests, statistical significance was declared at p < 0.05 (two-sided). The analyses were performed using MATLAB 7.10 (R2010a), The MathWorks and PASW Statistics 18, SPSS Inc. Data are presented in mean ± SD.
Results
Glucose disposal and microvascular responses to euglycaemic hyperinsulinaemia
Steady-state plasma glucose was achieved after overnight insulin infusion as shown in Fig. 1. Plasma glucose was clamped at 6.6 ± 0.9 mmol/l for the 17 euglycaemic–hyperinsulinaemic clamps. The profiles of glucose and plasma insulin concentrations are shown in Fig. 2. The M value, logSI, insulin concentration measures Ip,basal, Ip,priming and Ip,ss, blood glucose concentrations before the start of the clamp (BGstart) and during the last 30 min of the clamp (BGend), measures of capillary recruitment MBVbasal and MBV30 min, assessed from the basal and 30 min sequences respectively, and ΔMBV are shown in Table 2.
a Blood glucose (BG) concentrations during the clamp. Microvascular recruitment was assessed through CEU imaging at basal plasma insulin levels (t = −10 min) and at hyperphysiological insulin levels (t = 30 min). (b) Plasma insulin concentrations before and during the clamp. The time intervals used to compute the insulin measures are indicated as ‘basal’ for Ip,basal: concentration before the start of the clamp; as ‘priming’ for Ip,priming: concentration during the insulin priming, and as ‘ss’ for Ip,ss: concentration at steady-state during the clamp. All concentrations are shown as mean ± SD (n = 17)
Relationship between basal MBV and insulin sensitivity
The relationship between MBVbasal and logSI is shown in Fig. 3. The correlation between MBVbasal and logSI is 0.62 (p = 0.008) and the linear regression of logSI on MBVbasal indicates that MBVbasal is a statistically significant predictor of logSI (p = 0.008). To control for the potential effect of the basal plasma insulin concentration on MBVbasal, we performed a linear regression of logSI on MBVbasal and Ip,basal and MBVbasal remained statistically significant (p = 0.014). The partial correlation between logSI and MBVbasal is 0.60, almost identical to the original correlation. Likewise, controlling for age, body weight, HbA1c and number of years since the diagnosis of type 1 diabetes did not change the significance of MBVbasal as a predictor of logSI. The positive relationship between logSI and MBVbasal shows that the participants whose microvasculature was most expanded after the overnight insulin infusion were more insulin sensitive. This suggests that the ability to recruit capillaries at modest levels of peripheral hyperinsulinaemia is a potential determinant of the sensitivity of a participant to insulin.
Relationship between changes in MBV and insulin sensitivity during the clamp
The relationship between capillary recruitment induced by high concentrations of insulin attained during the clamp and logSI is shown in Fig. 4. The correlation between ΔMBV and logSI is −0.55 (p = 0.022). When performing a regression of ΔMBV on logSI, the variable logSI was significant in the linear model (p = 0.022), meaning that the insulin sensitivity is a significant predictor for the dependent variable ΔMBV. The model indicates that the more insulin sensitive patients, with higher MBV after the overnight insulin infusion, experienced a lower increase in MBV than the more insulin resistant patients. Controlling for age, body weight, HbA1c and number of years since diagnosis with type 1 diabetes did not change the significance of logSI as a predictor of ΔMBV.
The participants were divided into two groups using a K-means cluster analysis with two clusters based on standardised ΔMBV and logSI. The two groups obtained are shown in Fig. 5a and listed in Table 1. No significant differences were found between the two groups with respect to age, duration of type 1 diabetes, body weight, BMI, HbA1c measured prior to the clamp admission and complication index. Insulin sensitivity and ΔMBV for the two groups are shown in Fig. 5b and c respectively and we observed a clear separation between the two groups on insulin sensitivity and on ΔMBV. Group 1 (n = 7) was characterised by lower insulin sensitivity but greater capillary recruitment during the clamp, while Group 2 (n = 10) had higher insulin sensitivity, but lower capillary recruitment. The mean insulin sensitivity of the two groups, 0.016 and 0.042 μmol kg−1 min−1 (pmol/l)−1 for Groups 1 and 2 respectively, were statistically different as assessed by an unpaired t test (p = 0.0002). Likewise, the mean change in MBV in the two groups, 28.1% and −18.7% respectively, were statistically different from each other (unpaired t test p = 0.019). Therefore, capillary recruitment during the higher dose insulin infusion in type 1 diabetes was related to the insulin sensitivity of the participants. More precisely, lower insulin sensitivity was related to higher capillary recruitment (ΔMBV for Group 1 = 28.1 ± 32.0%, p = 0.060) while a higher sensitivity was associated with a decline in MBV (capillary derecruitment, ΔMBV for Group 2 = −18.6 ± 18.7%, p = 0.012).
MBV after 30 min of hyperinsulinaemia was not statistically different between the two groups (3.58 ± 1.26 dB for Group 1 and 2.87 ± 0.77 dB for Group 2, p = 0.170) and the correlation between logSI and MBV30min is almost null (r = 0.02, p = 0.95). Furthermore, MBV30min for Group 1 (3.58 ± 1.26 dB) was not statistically different from MBVbasal for Group 2 (3.61 ± 0.85 dB), indicating that microvascular recruitment in the more resistant group attained similar levels to that achieved in the sensitive group at basal levels of plasma insulin.
Discussion
We found that participants who were more insulin sensitive had a greater MBV after the overnight insulin infusion, while more insulin resistant participants increased MBV only after 30 min of marked hyperinsulinaemia. To emphasise the relationship between insulin sensitivity and relative change in MBV, the participants were grouped through a cluster analysis. The groups were thus defined based on the data rather than on arbitrary thresholds. The first group included more insulin resistant participants while the second group included more sensitive ones. The absolute MBV after the first 30 min of the insulin clamp was not statistically different between the two groups. Together, these observations suggest that insulin-induced capillary recruitment occurs at levels of plasma insulin that depend on the metabolic insulin sensitivity of the individual and, more precisely, that individuals whose microvascular volume is expanded after the overnight insulin infusion are those with higher whole-body glucose uptake during the steady-state of the euglycaemic clamp. Conversely, individuals for whom higher insulin concentrations are needed to induce capillary recruitment will not be as insulin sensitive.
We have previously reported a dose–response relationship between insulin-induced capillary recruitment and MBV in rodents. In those studies we observed that insulin infusions of 3 mU kg−1 min−1 produced near maximal capillary recruitment and increasing plasma insulin further did not further augment capillary opening [25]. Here we computed insulin sensitivity based on the steady-state glucose consumption during the final 30 min of the clamp and found that patients with higher MBV after overnight insulin infusion are the patients with higher insulin sensitivity. This suggests that greater vascular insulin sensitivity predicts greater metabolic insulin sensitivity. It is important to note that, at 30 min after the start of the clamp, the plasma insulin concentration reached steady-state levels and MBV was similar between the insulin sensitive and insulin resistant groups. If the effect of time on MBV is negligible compared with the effect of plasma insulin levels over the duration of the clamp, one would predict that, at the time at which insulin sensitivity was measured (final 30 min of the clamp), the endothelial surface available for insulin transfer to muscle would be similar, and differences in metabolic insulin sensitivity would be attributable to either delays in insulin crossing the endothelium [6] or resistance at the myocyte level per se [26].
We previously observed that a euglycaemic–hyperinsulinaemic clamp (insulin infusion rate = 1 mU kg−1 min−1) increased MBV (measured by CEU) in lean but not in obese humans [14]. MBV at baseline was comparable in the lean and the obese groups (18.7 ± 3.3 and 20.4 ± 3.6 respectively) despite higher plasma insulin concentrations in the obese. These obese participants were quite insulin resistant [27], as assessed from the whole-body glucose infusion rate during hyperinsulinaemia. Because type 1 diabetic patients require continuous insulin treatment, basal MBV in the current study corresponds to MBV measured after an overnight insulin infusion, while baseline MBV in the obese/lean study corresponds to MBV measured before the start of the clamp, with no insulin infusion. In consequence, the plasma insulin concentrations in the population with type 1 diabetes before (285.8 ± 132.4 pmol/l) and during the clamp (899.9 ± 331.0 pmol/l) were substantially higher than in either the obese or lean participants studied previously both before (91.7 ± 12.5 pmol/l for the obese and 43.8 ± 7.0 pmol/l for the lean) and during the clamp (≈450 pmol/l for the obese and 415 pmol/l for the lean (see Fig. 2a. in Clerk et al. [14]). Our study suggests that plasma insulin concentrations higher than 450 pmol/l might be needed to trigger capillary recruitment in the obese individuals.
Laakso et al. studied the insulin dose-response of leg blood flow in overnight fasted humans before and during insulin infusion [12]. Leg blood flow was similar in lean and obese participants in the postabsorptive state and increased about twofold in a sigmoidal fashion in both groups as a function of insulin concentration. Maximum leg blood flow rates were similar in obese and lean participants. However, the plasma insulin concentration, which half-maximally increased leg blood flow (ED50), was 3.6 times higher in obese participants than in lean participants (1107.7 vs 307.7 pmol/l), demonstrating vascular insulin resistance in these obese participants. We have not studied the insulin dose-response for MBV in obese humans. However, if the insulin dose-response curve for capillary recruitment parallels that for total blood flow, the higher ED50 for obese participants compared with lean participants would be consistent with the absence of insulin-induced capillary recruitment in the obese participants at the levels of plasma insulin observed in Prager et al. [27]. Interestingly, in the current study of type 1 diabetes we observed a negative correlation (r = −0.44, p = 0.081) between BMI and logSI in these type 1 diabetic participants, indicating that participants who were more insulin resistant were heavier in this population as well. A rightward shift for the dose-response of insulin-mediated capillary recruitment is consistent with the results obtained in our study.
In the blood circulation, insulin stimulates the release of both endothelin-1 (ET-1), a potent vasoconstrictor, and NO, a vasodilator, the proportion of one to the other determining the haemodynamic effect of insulin [28]. It is of interest that the insulin sensitive participants with type 1 diabetes not only failed to further increase MBV during the clamp but MBV on average declined. Others have pointed out that prolonged insulin infusion (>6 h) can actually lead to an inhibition of NO-mediated vasodilation [29]. In our study, the effect of insulin in increasing ET-1 may have become dominant when the insulin clamp followed the overnight infusion of insulin in the insulin sensitive group, but not in the resistant group.
In conclusion, we studied the action of insulin on muscle microvascular recruitment and its relation to metabolic insulin sensitivity in participants with type 1 diabetes. The data indicate that microvascular perfusion is positively related to insulin sensitivity, with insulin sensitive participants having an expanded MBV after an overnight insulin infusion, while 30 min of greater hyperinsulinaemia was required to recruit capillaries in more insulin resistant participants. These findings indicate that the moderate insulin resistance seen in type 1 diabetes extends to the vascular responsiveness to insulin and that vascular and metabolic insulin resistance are linked in type 1 diabetic individuals, as has been reported for obese insulin resistant individuals.
Abbreviations
- BGend :
-
Blood glucose at end of clamp
- BGstart :
-
Blood glucose at start of clamp
- CEU:
-
Contrast-enhanced ultrasound
- ET-1:
-
Endothelin-1
- HCTZ:
-
Hydrochlorothiazide
- Ip,basal :
-
Insulin concentration before start of clamp
- Ip,priming :
-
Insulin concentration during insulin priming
- Ip,ss :
-
Insulin concentration during final 30 min of clamp
- MBV:
-
Microvascular blood volume
- MBVbasal :
-
MBV at basal levels of insulin
- MBV30min :
-
MBV at high physiological levels of insulin
- ROI:
-
Region of interest
- SI:
-
Measure of insulin sensitivity
References
Baron AD, Brechtel-Hook G, Johnson A, Hardin D (1993) Skeletal muscle blood flow. A possible link between insulin resistance and blood pressure. Hypertension 21:129–135
Baron AD, Laakso M, Brechtel G, Edelman SV (1991) Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab 73:637–643
Baron AD (1994) Hemodynamic actions of insulin. Am J Physiol 267:E187–E202
Gudbjörnsdóttir S, Sjöstrand M, Strindberg L, Wahren J, Lönnroth P (2003) Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab 88:4559–4564
Bergman RN (2003) Insulin action and distribution of tissue blood flow. J Clin Endocrinol Metab 88:4556–4558
Barrett EJ, Eggleston EM, Inyard AC et al (2009) The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52:752–764
Yang YJ, Hope ID, Ader M, Bergman RN (1989) Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Investig 84:1620–1628
Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ (2004) The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metabol Res Rev 20:3–12
Vincent MA, Dawson D, Clark ADH et al (2002) Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51:42–48
Coggins M, Lindner J, Rattigan S et al (2001) Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50:2682–2690
Rattigan S, Clark MG, Barrett EJ (1997) Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 46:1381–1388
Laakso M, Edelman SV, Brechtel G, Baron AD (1990) Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Investig 85:1844–1852
Vincent MA, Clerk LH, Lindner JR et al (2004) Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53:1418–1423
Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ (2006) Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55:1436–1442
Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S (2003) Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285:E123–E129
Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ (2009) Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32:1672–1677
Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ (2009) Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94:3543–3549
DeFronzo RA, Hendler R, Simonson D (1982) Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 31:795–801
Nadeau KJ, Regensteiner JG, Bauer TA et al (2010) Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 95:513–521
Mahmud FH, van Uum S, Kanji N, Thiessen-Philbrook H, Clarson CL (2008) Impaired endothelial function in adolescents with type 1 diabetes mellitus. J Pediatr 152:557–562
Peña AS, Wiltshire E, Gent R, Hirte C, Couper J (2004) Folic acid improves endothelial function in children and adolescents with type 1 diabetes. J Pediatr 144:500–504
DeFronzo R, Tobin J, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237:E214–E223
Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S (1998) Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97:473–483
Chan A, Kovatchev BP, Anderson SM, Breton MD (2011) Systematic method to assess microvascular recruitment using contrast-enhanced ultrasound. Application to insulin-induced capillary recruitment in subjects with T1DM. Comput Meth Programs Biomed 102:219–226
Zhang L, Vincent MA, Richards SM et al (2004) insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53:447–453
Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S (1994) Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Investig 93:10–16
Prager R, Wallace P, Olefsky JM (1986) In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Investig 78:472–481
Cardillo C, Nambi SS, Kilcoyne CM et al (1999) Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 100:820–825
Arcaro G, Cretti A, Balzano S et al (2002) Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation 105:576–582
Acknowledgements
This study is supported by the NIH/NIDDKRO1 DK 51562 grant and the University of Virginia General Clinical Research Center M01 RR 000847 grant.
Contribution statement
All the authors, AC, EJB, SMA, BPK and MDB, contributed to the conception and design, or analysis and interpretation of the data, the drafting the article or revising it critically for important intellectual content, and the final approval of the version to be published.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, A., Barrett, E.J., Anderson, S.M. et al. Muscle microvascular recruitment predicts insulin sensitivity in middle-aged patients with type 1 diabetes mellitus. Diabetologia 55, 729–736 (2012). https://doi.org/10.1007/s00125-011-2402-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2402-3