Abstract
Aims/hypothesis
NOD.Igµ null mice lacking mature B cells are highly resistant to diabetes and display poor CD4 T cell responses to autoantigens. Nevertheless, the degree to which different B cell subsets contribute to diabetes in NOD mice remains unresolved. Due to their role in the recognition of microbial and autoantigens, peritoneal B cell characteristics were examined in NOD mice to see if they differ developmentally, phenotypically or functionally in aspects relevant to diabetogenesis.
Methods
The population dynamics, activation state, migratory behaviour and antigen presentation function were investigated in NOD peritoneal B cells.
Results
NOD peritoneal B cells were found to express abnormally high levels of co-stimulatory molecules (CD40, CD86 and CD69). In contrast, the expression of l-selectin and integrin α4β1 was markedly reduced in NOD mice compared with BALB/c and C57BL/6 mice. The number of B cells in the peritoneum was lower in NOD than in control mice throughout development; migration of B cells from the peritoneum to the pancreatic lymph nodes in NOD mice was enhanced tenfold. NOD B cells showed no chemotactic response to sphingosine-1-phosphate, which normally acts to retain B cells in the peritoneum. Peritoneal B cells of NOD mice also presented insulin autoantigen to CD4 T cells, inducing T cell proliferation.
Conclusions/interpretation
NOD peritoneal B cells are hyperactivated, migrate to the pancreatic lymph nodes and are capable of driving insulin-specific CD4 T cell activation. These characteristics could make them important for inducing or amplifying T cell responses against islet-antigens.
Similar content being viewed by others
Introduction
The importance of B cells for the development of type 1 diabetes in NOD mice has become increasingly evident over the last 10 years [1–6]. Several reports have demonstrated that eliminating B cells protects against type 1 diabetes in mice [1, 3, 6]. B1 cells moreover produce natural antibodies and recognise common bacterial antigens as well as self-antigens [7]. The specific role of NOD marginal zone B cells has been evaluated in a type 1 diabetes context [4, 5]. These B cells expand with the onset of diabetes and are able to present insulin peptides to diabetogenic T cells [5].
Peritoneal B cells, however, have not as yet been researched to the same extent as other B cell subsets. Since peritoneal B cells comprise an exceptionally high concentration of B1 type B cells, which are of particular interest in autoimmunity, a thorough examination of the role of peritoneal B cells in type 1 diabetes is paramount. Nonetheless, some evidence exists for the importance of peritoneal B cells, and more specifically of the peritoneal B1 cell subset, in type 1 diabetes. Many islet-infiltrating B cells in NOD mice express the CD5 antigen, which is also expressed by most B1 cells [6]. These B1-like B cells have been reported to decrease in number in infiltrates following hypotonic lysis of peritoneal cells. The depletion of peritoneal B cells also protects NOD mice from type 1 diabetes [6]. Insulin autoantibodies measured by competitive radiobinding assays are typically of high affinity in humans and in NOD mice [8, 9]. However, low-affinity insulin-binding autoantibodies that interact with multiple antigens have been reported in young NOD mice [10]. Thus some of the early repertoire of insulin autoantibodies in NOD mice appear to share characteristics with natural antibodies. B1 cells could hence be implicated as producers of autoreactive antibodies such as insulin autoantibodies and in the presentation of insulin- and/or other islet-antigens to diabetogenic T cells. This would, however, require that they endocytose islet-antigens and provide co-stimulatory signals for T cells.
Sphingosine-1-phosphate (S1P) has received considerable attention in recent years as a regulator of lymphocyte trafficking. In the peritoneum, S1P helps retain B lymphocytes within the peritoneal cavity [11]. B cell unresponsiveness to chemotactic signals from S1P thus facilitates the emigration of peritoneal lymphocytes. An analogous modulator of peritoneal cell migration is integrin α4β1. High expression of this integrin on B1 lymphocytes keeps these lymphocytes in the peritoneal cavity [12]. Activating signals such as lipopolysaccharide have the effect of impeding S1P signalling [13] as well downregulating expression of integrin α4β1, consequently permitting B cell exit.
An interesting aspect of the peritoneal B cell subset is its location adjacent to, and interrelation with the intestine. Peritoneal B cells in mice respond to microbial and inflammatory signals derived from the gastrointestinal tract [12]. Moreover, Turley and collegues have suggested the existence of an inherent trafficking route between the peritoneum and the pancreatic lymph nodes (PaLN) [14]. This provides a unique opportunity for peritoneal B cells to directly interlink environmental and microbial factors with autoimmune manifestations in the PaLN.
Methods
Mice
Non-obese diabetic mice were used at 5 to 10 weeks, unless otherwise indicated; age-matched BALB/c and C57/Bl6 mice were used as controls. The mice were raised and bred in the Central Animal Laboratory of Turku University. All animal experiments were approved by the National Laboratory Animal Care and Use Committee in Finland and conformed to the legal acts, regulations and requirements of the European Union concerning the protection of animals used for research.
Cell isolation
Peritoneal cells were obtained by washing out the peritoneum with cold RPMI-1640 medium (Gibco, Grand Island, NY, USA). Single-cell suspensions of spleen cells and lymph node cells were acquired by gently pressing the tissues through a metal mesh. Erythrocytes were extracted by hypotonic lysis using 0.2% (wt/vol.) NaCl.
Immunofluorescence and flow cytometry
To assess B cell types and numbers, peritoneal cells were incubated either with biotinylated anti-CD45R (R&D Systems, Minneapolis, MN, USA) followed by incubation with streptavidin–allophycocyanin (Invitrogen, Carlsbad, CA, USA), or with allophycocyanin-conjugated anti-CD45R (Caltag Laboratories, Burlingame, CA, USA). To distinguish between B1 and B2 cells, either CD11b FITC (which stains B1a and B1b type B1 cells) (Immunotools, Friesoythe, Germany) or CD5 PE (for staining of B1a cells) (R&D systems) were used. After surface staining, cells were analysed using FACS Calibur. To examine the surface expression of various activation markers and integrins, the following PE-conjugated antibodies were used in addition to anti-CD45R and CD11b: CD40 (BD Pharmingen, Franklin Lakes, NJ, USA), CD86, Integrin α4 (BD Biosciences, San Jose, CA, USA, and AbD Serotec, Oxford, UK), Integrin β1 (AbD Serotec), Integrin α4 β7 heterodimer (DATK32; BD Pharmingen) and CD69 (Immunotools). The L-selectin antibody that was used was FITC-conjugated (Immunotools). S1P receptor 1 surface expression analysis was performed using rabbit anti-endothelial differentiation sphingolipid G-protein-coupled receptor 1 (EDG1) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Alexa-fluor 488-labelled anti-rabbit IgG (Invitrogen) as the second-step antibody. Flow cytometry was performed using FACS Calibur (BD Biosciences) and Cell Quest (BD) software.
Adoptive transfer experiments
Peritoneal cells from BALB/c and NOD mice were labelled with Vybrant DiI dye (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s recommendations. We injected 2 × 106 cells intraperitoneally into each recipient mouse. The mice were killed 20 h later and peritoneal cells, mesenteric lymph nodes, spleens and PaLN were isolated. The cells obtained were stained with fluorochrome-conjugated CD45R and CD11b antibodies. Frequencies of adoptively transferred B cells in collected samples were analysed by flow cytometry and the results expressed as ‰ of total (DiI-negative) B cells collected for each sample of mesenteric lymph nodes, spleen and PaLN. The decrease in DiI-labelled B cells in the peritoneum at 20 h after transfer was determined by measuring DiI + B cells as percentage of total DiI + cells at the time of transfer vs 20 h post-transfer. Results are expressed as per cent DiI-labelled B cells left in peritoneum relative to input.
In vitro cell migration experiments
Migration experiments were performed using 96-well Transwell Permeable Supports (Corning, Corning, NY, USA) with 5 μm pore sizes. Peritoneal cells from NOD and BALB/c mice were isolated. Macrophages were removed by adherence to plastic for 1 to 2 h in RPMI medium containing 10% FCS. This step typically yielded a cell suspension with approximately 90% purity of B cells. Serum-free medium supplemented with 0.1% fatty acid free BSA was added to the lower wells with different concentrations (1, 5, 10, 30, 100, 500 or 1,000 nmol/l) of S1P (Biomol International, Plymouth, UK) or without S1P (control). The enriched B cells were washed in RPMI, resuspended in serum-free medium supplemented as described above and added to the upper wells (75,000 cells/well). In some experiments, B cells were pre-incubated for 1 h with 1 μmol/l VPC23019 (Avanti Polar Lipids, Alabaster, AL, USA), an antagonist of S1P receptors 1 and 3. VPC23019 was additionally added at 1 μmol/l to the upper and lower wells of the migration assay in these experiments. Cell migration was allowed to proceed for 1 h at 37ºC. Cells that had migrated to the lower wells were then collected and quantified by flow cytometry. Quantification was based on cell counts obtained by flow cytometry during a set time constant (100 s) with identical cell-suspension volumes. The margin of error using this method of analysis was approximately 10%, which was roughly the same as when using TruCount fluorescent beads for quantification with flow cytometry.
Western blotting
Peritoneal cells from NOD and BALB/c mice were isolated. Macrophages were removed by adherence to plastic as described above. Whole-cell lysates from B cell-enriched peritoneal cells were obtained by incubating the cells on ice for 1 h, under agitation in a lysis buffer containing 20 mmol/l Tris base, 150 mmol/l NaCl, 0.5% (vol./vol.) Nonidet P-40, 2 mmol/l EDTA, 1 mmol/l phenylmethylsulphonyl fluoride and aprotinin 0.01 trypsin inhibitor units per ml (pH 8.0). The cell suspension was then centrifuged at 15,000×g for 15 min at 4ºC, after which the supernatant fraction was collected. The protein concentration in the sample was determined with a BSA protein assay reagent kit (Pierce, Rockford, IL, USA) according to the manufacturer’s recommendations.
To determine peritoneal cell S1P receptor 1 and 3 levels, protein lysates were mixed 3:1 in 3× SDS buffer (30% [vol./vol.] glycerol, 3% [wt/vol.] SDS, 187.5 mmol/l Tris/HCl, pH 6.8, 0.015% (wt/vol.) Bromophenol Blue and 3% [wt/vol.] 2-mercaptoethanol). The mixture was then heated for 4 min at 95°C. Western blotting was performed in accordance with Balthasar et al. [15]. Rabbit anti-EDG1 and rabbit anti-EDG3 (Santa Cruz Biotechnology) were used as primary antibodies and HRP-conjugated goat anti-rabbit IgG (BioRad Laboratories, Hercules, CA, USA) as the secondary antibody. Finally, membranes were stripped as described [15] and secondary probing with the loading control β-actin (Cell Signalling Technology, Danvers, MA, USA) was performed. Densitometric analysis of the blots was performed using ImageJ 1.41 software (US Department of Health and Human Services, USA; http://rsbweb.nih.gov/ij/) and S1P receptor blot intensities were correlated to the β-actin loading control blot intensities.
In vitro S1P-induced CD69 downregulation
Peritoneal cells were incubated overnight in serum-free medium supplemented with 0.1% fatty acid free BSA, 2 mmol/l l-glutamine and 100 units/ml penicillin and streptomycin, either in the presence or in the absence of S1P (200 nmol/l). The cells were collected and incubated with antibodies against B220/CD45R, CD11b and CD69 and analysed using flow cytometry.
In vitro spleen and peritoneal cell proliferation assay
Peritoneal and spleen cells from BALB/c and NOD mice were labelled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) (5 μmol/l; Molecular Probes), diluted to a concentration of 1 × 106 cells/ml and cultured for 72 h on 96-well culture plates in culture medium alone or with the addition of anti-mouse CD40 antibody (3 μg/ml; BD Biosciences), CpG oligodeoxynucleotide (CpG) (1 μg/ml; CpG 1826; Coley Pharmaceutical, Langenfeld, Germany) or lipopolysaccharide (10 μg/ml; Sigma-Aldrich, St Louis, MO, USA). Finally, cells were collected from the wells, stained with anti CD45RB/B220-APC and CD5-PE conjugated antibodies, and subjected to flow cytometric analysis.
Antigen presentation assay
NOD and BALB/c mice were immunised subcutaneously on the hind flank with 50 μg insulin peptide (Insulin B [9–23]; Anaspec, San Jose, CA, USA). After 10 days, spleens were collected from these animals and splenic CD4+ T cells purified using CD4 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). These purified T cells were co-cultured (200,000 cells/well) with peritoneal or splenic B cells (150,000 cells/well) purified with B220 MicroBeads (Miltenyi Biotech). Additionally, either insulin peptide (4 μmol/l or 40 μmol/l; Insulin B 9-23; Anaspec) or intact insulin (20 μg/ml or 200 μg/ml; Sigma-Aldrich) was added to some of the wells. The cells were incubated for 72 h at 37ºC with the addition of [3H]thymidine (148 kBq/ml) during the last 16 h of incubation. Finally, cells were collected using an automatic cell harvester (Harvest 96; Tomtec, Frankfurt, Germany) and radioactivity was counted using a beta counter (Wallac, San Diego, CA, USA). Each experiment was performed in triplicate.
Statistical analysis of the data
The results are presented as averages of at least three independent experiments ± SEM. All individual in vitro cell migration experiments were performed in duplicates. The Student’s t test was used for comparison between two groups. One-way ANOVA and relevant post-hoc tests were used, if more than two groups were compared.
The results for the adoptive transfer experiments are presented as the fraction of DiI-labelled B cells found in the PaLN divided by the fraction of DiI-labelled cells in the peritoneum. This formula was employed to minimise any errors arising from potential variations in cell numbers injected into recipients.
Results
The peritoneal B cell pool develops poorly in NOD mice
The numbers of B1 and B2 cells increased rapidly in the peritoneum of BALB/c mice between 4 and 9 weeks of age, with numbers of the former continuing to increase until a mature age (>17 weeks). In contrast, B1 cell numbers in NOD mice remained at a constantly low level throughout development of the animals, showing only a slight increase upon reaching maturity (>17 weeks) (Fig. 1). B2 cell numbers were also significantly lower in the NOD peritoneum between 4 and 13 weeks of age. However, numbers of B2 cells in NOD mice reached those of BALB/c mice by week 17 (Fig. 1a, b).
The peritoneal B cell pool develops poorly in NOD mice. a Peritoneal B1 cell numbers and (b) peritoneal B2 cell numbers as a function of age in NOD (white circles) and BALB/c (black squares) mice between 3 and >17 weeks of age, n = 3–6 mice per group; *p < 0.05, **p < 0.01, ***p < 0.001 for difference between NOD and BALB/c mice of the same age
In vivo migration of NOD peritoneal cells
Labelled peritoneal cells from NOD and BALB/c mice were transferred intraperitoneally to intact recipients of the same strain. The transferred cells migrated preferentially to the PaLN via spleen and mesenteric lymph nodes in NOD mice (Fig. 2b). The migration rate of peritoneal B cells to PaLN was enhanced tenfold in NOD mice compared with BALB/c mice (Fig. 2a, b). In line with this, the percentage of DiI-labelled B cells left in the peritoneum 20 h after transfer was significantly lower in NOD mice.
Peritoneal B cells of NOD mice accumulate in PaLN in vivo following adoptive transfer. Peritoneal cells (2 × 106) were labelled and injected intraperitoneally into recipient mice in each experiment. a Dotplots of DiI-labelled B cells (DiI+ B220+, top right quadrant) in PaLN of NOD and BALB/c mice, with adoptive transfer from a NOD mouse to a NOD recipient and from a BALB/c mouse to a BALB/c recipient. b Fraction of DiI-labelled peritoneal B cells retrieved from PaLN, spleen and mesenteric lymph nodes (MLN) in NOD (black bars) and BALB/c (white bars). n = 5 mice per group; ***p < 0.001 as calculated with one-way ANOVA and Bonferroni’s multiple comparisons test. c Fraction of DiI-labelled B cells left in the peritoneum 20 h post transfer. n = 5 mice per group; *p < 0.05
Defective integrin expression and S1P responsiveness in NOD peritoneal B cells
NOD peritoneal B cells did not increase with age during adolescence and showed an enhanced tendency to migrate out of the peritoneum (to the PaLN). Since peritoneal cell migration into/out of the peritoneal cavity is regulated in part by integrins, the expression of integrin α4 (CD49d) and integrin β1 (CD29) on peritoneal B cells was investigated. The level of integrin α4 was significantly lower on NOD B1 cells than on B1 cells from C57BL/6 or BALB/c mice. The downregulation of integrin α4 in NOD mice appears to occur prior to insulitis, since it was observed from 3 weeks onwards (Fig. 3).
Integrin α4β1 expression and S1P-induced in vitro migration of peritoneal B cells. a Integrin α4 mean fluorescence intensity (MFI) of 5- to 10-week old C57BL/6 (black bar), BALB/c (grey bar) and NOD (white bar) B1 cells. b Integrin α4 MFI of 3-week old BALB/c (grey bar) and NOD (white bar) B1 cells. n = 3. c Representative dotplots showing integrin α4 and β1 expression on BALB/c and NOD B1 cells as indicated. d In vitro migration of NOD (white circles) and BALB/c (black squares) peritoneal B cells towards different concentrations of S1P. n = 3–6. e Migration of NOD (white bars) and BALB/c (black bars) peritoneal B cells towards medium alone (Control) or 10 nmol/l S1P. Checked bar represents migration of BALB/c peritoneal B cells towards 10 nmol/l S1P in the presence of 1 μmol/l of the inhibitor VPC23019, n = 3. *p < 0.05, ***p < 0.001
Moreover, NOD B1 cells underexpressed integrin β1 (Fig. 3c). Given that peritoneal B cells did not express integrin α4β7 in any of the mouse strains (not shown), we conclude that the heterodimer integrin α4β1 is dysregulated on peritoneal B1 cells of NOD mice. Peritoneal B2 cells were also investigated, but did not express integrin α4 in any of the three mouse strains.
S1P has received considerable attention in recent years as a regulator of lymphocyte trafficking [16]. For peritoneal B lymphocytes, S1P signalling is required for retention of B-lymphocytes within the peritoneum [11]. In agreement with a previous study [12], a moderate but significant migratory response of BALB/c peritoneal B cells towards S1P was observed (Fig. 3d). The migration rate peaked at 10 nmol/l S1P and returned to control levels when the S1P concentration reached 100 nmol/l, demonstrating a bell-shaped dose–response curve that is typical of chemokine receptor-mediated cell migration. Moreover, pre-incubation with the S1P receptor 1 and S1P receptor 3 inhibitor, VPC23019, completely abolished the migratory response of BALB/c peritoneal B cells towards 10 nmol/l S1P (Fig. 3e). However, NOD peritoneal B cells did not significantly respond to S1P in in vitro migration assays at any of the S1P concentrations (1, 10, 300, 500 or 1,000 nmol/l) (Fig. 3d).
S1P receptors and CD69 expression in NOD peritoneal B cells
To investigate the impaired responsiveness of NOD peritoneal B cells to S1P, the level of S1P receptor 1 and S1P receptor 3 was examined by western blotting of whole-cell lysates from NOD and BALB/c peritoneal B cells. S1P receptor 3 levels were roughly equal in the two mouse strains, but S1P receptor 1 levels were higher in NOD peritoneal B cells (Fig. 4a, b). Yet, when surface expression of S1P receptor 1 was examined by flow cytometry, NOD peritoneal B cells expressed less S1P receptor 1 than BALB/c peritoneal B cells, although the difference was not statistically significant (Fig. 4d). Shiow and colleagues [17] have demonstrated that high levels of CD69 downregulate S1P receptor 1 surface expression without substantially affecting S1P receptor mRNA expression. As seen in Fig. 5c, NOD peritoneal B cells exhibited exceptionally high levels of CD69, but overnight in vitro stimulation with 200 nmol/l S1P induced a 30% downregulation of CD69 (Fig. 4e).
S1P receptor 1 (S1P1) and S1P receptor 3 (S1P3) expression, and S1P-induced downregulation of CD69. a Representative western blots of S1P1 and S1P3 receptors (S1PR) in NOD and BALB/c peritoneal B cells and the loading control β-actin. b Densitometrically quantitated values of western blots of S1P1 and (c) S1P3 expression in NOD and BALB/c peritoneal B cells normalised to the loading control β-actin; n = 3. d Mean fluorescent intensity (MFI) of S1P1 surface expression on NOD (black bar) and BALB/c (white bar) peritoneal B cells analysed using flow cytometry; n = 3 mice/group. e S1P-induced CD69 downregulation on NOD peritoneal B cells. Black bar, normalised MFI values of CD69 expression levels on control cells (i.e. NOD peritoneal B cells incubated in vitro overnight without S1P); white bar, values on NOD peritoneal B cells after overnight in vitro stimulation with 200 nmol/l S1P; n = 7. *p < 0.05, ***p < 0.001
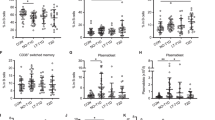
Expression of co-stimulatory molecules on peritoneal B cells. a, b Mean fluorescent intensity (MFI) of CD40 and (c, d) CD86 on peritoneal B1 cells (a, c) and B2 cells (b, d). e, f Per cent of CD69- and l-selectin- (g, h) positive peritoneal B1 cells (e, g) and B2 cells (f, h). Black bars, B cells from NOD mice; white bars, B cells from BALB/c mice; checked bars, B cells from C57BL/6 mice. All cells were labelled with B220 and CD11b to distinguish B1 and B2 cells. *p < 0.05 and **p < 0.01 (Holm–Bonferroni method) for differences between NOD and BALB/c, and NOD and C57BL/6 mice; n = 3–6 mice/group. i CD69 expression on peritoneal B1 and (j) B2 cells from NOD (black circles) and BALB/c (white squares) at indicated times. k l-selectin expression on peritoneal B1 and (l) B2 cells from NOD and BALB/c mice at indicated times. n = 3–6 mice/group. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test)
Expression of co-stimulatory molecules on NOD peritoneal B cells
Peritoneal B cells from 5- to 10-week old NOD mice expressed higher levels of several activation markers compared with BALB/c and C57BL/6 mice of the same age (Fig. 5a–h). Levels of the co-stimulatory receptor CD40 was significantly higher on B1 as well as B2 cells from NOD than on those from BALB/c and C57BL/6 mice. CD86, which could only be detected on B1 cells, was also overexpressed in the NOD mouse.
The majority (56%) of NOD B2 cells expressed CD69, as compared with 24% of C57BL/6 B2 cells and only 8% of B2 cells from BALB/c mice. A smaller, yet significant increase in CD69 expression was also observed on NOD B1 cells. Less than 30% of NOD B1 cells expressed l-selectin, as compared with over 75% of BALB/c and C57BL/6 B1 cells. There was no significant difference in l-selectin expression between B2 cells from NOD, BALB/c and C57BL/6 mice.
Expression of CD40, CD86, CD69 and l-selectin was additionally analysed in 3-week old mice. CD40 and CD86 were not yet upregulated in NOD mice at this time point (result not shown), but CD69 was upregulated and l-selectin downregulated. Moreover, the increased CD69 expression and decreased l-selectin expression was still evident in NOD mice at >17 weeks of age (Fig. 5i-l).
NOD peritoneal B cells respond strongly to CD40 ligation
To evaluate the responsiveness of NOD peritoneal B cells to stimulation through CD40, toll-like receptor (TLR) 4 or TLR 9, the proliferative responses of peritoneal B cells and splenic B cells to anti-CD40 antibody, lipopolysaccharide and CpG were compared (Fig. 6a). There was no significant difference in the response of NOD peritoneal or splenic B cells to CpG, lipopolysaccharide stimulation or CD40 ligation. Nevertheless, the response of NOD peritoneal B cells to CD40 ligation was stronger than that of BALB/c peritoneal B cells (Fig. 6b).
Peritoneal B cells are hyper-responsive to CD40-ligatiosn but not to CpG or lipopolysaccharide stimulation. a Representative histograms showing the reduction of CFSE-intensity after stimulating NOD peritoneal B1 (CD5+) and B2 (CD5−) cells or splenic B cells with 3 μg/ml CD40 antibody, 1 μg/ml CpG or 10 μg/ml lipopolysaccharide (LPS). Black histograms, control cells; white histograms, stimulated cells. n = 3. b Representative histograms showing proliferation of BALB/c and NOD peritoneal B cells as labelled in response to CD40 ligation. Grey histograms, control cells; white histograms, stimulated cells. c Per cent reduction in mean fluorescent intensity of CFSE-labelled cells after CD40 ligation on NOD and BALB/c peritoneal B cells. **p < 0.01
Antigen-presenting capacity of peritoneal B cells
The expression of co-stimulatory molecules (e.g. CD40 and CD86) was abnormally high on NOD peritoneal cells. Moreover, these cells reacted vigorously to CD40 ligation. These properties are paramount for efficient antigen presentation. Indeed, peritoneal B cells from NOD mice did prove more efficient at promoting T cell proliferation in response to insulin B9-23 and intact insulin than splenic B cells (Fig. 7). In contrast, peritoneal B cells from BALB/c mice did not induce T cell proliferation any more efficiently than BALB/c splenic B cells. These results emphasise that the heightened activation status is typical of the peritoneal B cell subset in NOD mice.
Autoantigen presentation by peritoneal and splenic B cells. B cell-induced T cell proliferation is shown using purified NOD or BALB/c peritoneal B cells (black bars) or splenic B cells (white bars). Grey bars, control values. Peritoneal cells were pooled for each experiment from four mice to yield sufficient numbers of purified B cells. Splenic B cells were pooled and purified from the same donors. a NOD T cell proliferation in response to insulin peptide B 9-23-stimulated B cells. b NOD T cell proliferation in response to B cells stimulated with intact insulin. c BALB/c T cell proliferation in response to insulin peptide. d BALB/c T cell proliferation in response to insulin. Baseline cpm range 30–200 cpm. Data represents mean values ±SEM of triplicate samples from two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 calculated with one-way ANOVA and Dunnett’s post hoc test
Discussion
The reduced number of B cells in the peritoneum of NOD mice appears to correlate with a strongly accelerated migration rate of these cells specifically to the PaLN. The results presented here show a tenfold increase in the migration rate of peritoneal B cells to PaLN in NOD mice, compared with BALB/c mice.
The retention of B1 cells in the peritoneal cavity is in part dependent on the expression of integrin α4β1. This is demonstrated by the rapid clearance of peritoneal B1 cells in reponse to lipopolysaccharide-stimulated downregulation of integrin α4β1 [18]. Integrin α4β1 was found to be markedly downregulated on NOD peritoneal B1 cells from a very early age. Low expression of integrin α4β1 on NOD B1 cells, in addition to being indicative of cell activation, undoubtedly contributes to the enhanced egress of B1 cells from the peritoneum in NOD mice.
The function of S1P in the peritoneal cavity is to retain B cells in the peritoneum. [11]. Insensitivity to S1P would thus allow increased migration of these cells out of the cavity. In contrast to BALB/c peritoneal B cells, NOD peritoneal B cells showed no in vitro migratory response to S1P. The reason for this was presumed to be either a lack of S1P receptors 1 and/ or 3, which are known to communicate the migratory response [13, 19], or alternatively a functional defect in signalling through these receptors. NOD peritoneal B cells were not in fact deficient in S1P receptor 1 or S1P receptor 3 proteins. However, when the surface expression of S1P receptor 1 was examined, it was found to be lower on NOD than on BALB/c peritoneal B cells. This could be explained by a cross-communication between CD69 and S1P receptor 1 [17]. High levels of CD69 downregulate S1P receptor 1 surface expression specifically, without substantially affecting S1P receptor mRNA expression. Moreover, high levels of CD69 functionally inhibit S1P receptor 1, causing cells to lose their chemotactic response to S1P [17]. The cross-communication between S1P receptor 1 and CD69 appears to be reciprocal, since stimulation with S1P downregulates CD69 on NOD T cells [20]. The same effect was observed on NOD peritoneal B cells. It is therefore suggested that NOD peritoneal B cell migration towards S1P is probably impeded by cross-communication between CD69 and S1P receptor 1 on the B cell surface, rather than by a lack of S1P receptor 1 proteins.
High surface expression of the co-stimulatory molecules CD40, CD86 and CD69, low l-selectin expression and a strong response to CD40 ligation demonstrate that NOD peritoneal B cells are highly activated. Moreover, co-stimulatory molecules are important factors in the pathology of diabetes. NOD T cells expressing high levels of CD40 are capable of inducing clinical diabetes [21], while CD86 upregulation on islet infiltrating NOD B cells is associated with development of insulitis [22]. CD69 is an early activation marker, but its sustained upregulation has been associated with a number of inflammatory and autoimmune diseases [23]. The anomalous levels of CD69 and l-selectin were observed on NOD peritoneal B cells from 3 weeks of age. This indicates that the increase in NOD peritoneal B cell activation status is initiated prior to insulitis onset.
The discovery that NOD peritoneal B2 and B1 cells express higher levels of various activation markers was unexpected, since the importance of peritoneal B cells in type 1 diabetes was thought to depend mostly on the B1 cell subset [24]. However, peritoneal B2 cells appear to constitute a unique B cell subset, which exhibits characteristics that are intermediate between peritoneal B1 cells and splenic B2 cells. Peritoneal B2 cells additionally acquire certain characteristics of B1 B cells upon residing in the peritoneal cavity [25]. CD11b, moreover, has been suggested to distinguish different developmental stages of peritoneal B cells [26] and its expression depends upon the location within the peritoneal cavity [27].
Previous studies have highlighted the importance of B cells as antigen-presenting cells in the pathology of diabetes [5, 24, 28, 29]. Our work demonstrates that peritoneal B cells are indeed capable of triggering a T cell response. In fact, peritoneal B cells seem to activate T cells more efficiently than splenic B cells. No distinction in antigen-presenting capacity between peritoneal and splenic B cells was observed in BALB/c B cells, underlining the exceptional characteristics of the NOD peritoneal B cell subset in this respect.
Peritoneal B cells reportedly interrelate with the immune system of the intestine and react to events taking place there [12]. In the study by Ha and co-workers [12], indomethacin was used to disrupt the gut epithelial barrier, resulting in downregulation of integrin α4 and an efflux of peritoneal B cells. Ha and co-workers [12] further reported that TLR stimulation causes upregulation of CD69 and decreased peritoneal B cell sensitivity to S1P chemotactic signals. All of these activation-related events coincide with the findings presented here regarding NOD peritoneal B cells. It is thus interesting to speculate whether the abnormalities observed in NOD peritoneal B cells are a consequence of disrupted gut epithelial barrier properties and/or TLR-stimulated events.
This study demonstrates that NOD peritoneal B cells are activated and traffic vigorously to the PaLN. Peritoneal B cells, moreover, efficiently present insulin autoantigen to self-reactive T cells, triggering T cell proliferation in vitro. These characteristics underline the role of peritoneal B cells in inducing or accentuating islet-specific autoimmune responses in NOD mice.
Abbreviations
- CFSE:
-
5,6-Carboxyfluorescein diacetate succinimidyl ester
- CpG:
-
CpG oligodeoxynucleotide
- EDG1:
-
Endothelial differentiation sphingolipid G-protein-coupled receptor 1
- PaLN:
-
Pancreatic lymph nodes
- S1P:
-
Sphingosine-1-phosphate
- TLR:
-
Toll-like receptor
References
Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A (1997) B cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes 46:941–946
Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM (1998) B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol 161:3912–3918
Hu CY, Rodriguez-Pinto D, Du W et al (2007) Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 117:3857–3867
Puertas MC, Carrillo J, Pastor X et al (2007) Phenotype and functional characteristics of islet-infiltrating B cells suggest the existence of immune regulatory mechanisms in islet milieu. Diabetes 56:940–949
Marino E, Batten M, Groom J et al (2008) Marginal-zone B cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T cells. Diabetes 57:395–404
Kendall PL, Woodward EJ, Hulbert C, Thomas JW (2004) Peritoneal B cells govern the outcome of diabetes in non-obese diabetic mice. Eur J Immunol 34:2387–2395
Kantor AB, Herzenberg LA (1993) Origin of murine B cell lineages. Annu Rev Immunol 11:501–538
Ziegler AG, Vardi P, Ricker AT, Hattori M, Soeldner JS, Eisenbarth GS (1989) Radioassay determination of insulin autoantibodies in NOD mice. Correlation with increased risk of progression to overt diabetes. Diabetes 38:358–363
Yu L, Robles DT, Abiru N et al (2000) Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 97:1701–1706
Thomas JW, Kendall PL, Mitchell HG (2002) The natural autoantibody repertoire of nonobese diabetic mice is highly active. J Immunol 169:6617–6624
Kunisawa J, Kurashima Y, Gohda M et al (2007) Sphingosine 1-phosphate regulates peritoneal B cell trafficking for subsequent intestinal IgA production. Blood 109:3749–3756
Ha SA, Tsuji M, Suzuki K et al (2006) Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med 203:2541–2550
Cinamon G, Matloubian M, Lesneski MJ et al (2004) Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol 5:713–720
Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C (2005) Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A 102:17729–17733
Balthasar S, Samulin J, Ahlgren H et al (2006) Sphingosine 1-phosphate receptor expression profile and regulation of migration in human thyroid cancer cells. Biochem J 398:547–556
Schwab SR, Cyster JG (2007) Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol 8:1295–1301
Shiow LR, Rosen DB, Brdickova N et al (2006) CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544
Lenschow DJ, Ho SC, Sattar H et al (1995) Differential effects of anti-B7–1 and anti-B7–2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med 181:1145–1155
Matloubian M, Lo CG, Cinamon G et al (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355–360
Srinivasan S, Bolick DT, Lukashev D et al (2008) Sphingosine-1-phosphate reduces CD4+ T cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes 57:484–493
Wagner DH Jr, Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K (2002) Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc Natl Acad Sci U S A 99:3782–3787
Hussain S, Delovitch TL (2005) Dysregulated B7-1 and B7-2 expression on nonobese diabetic mouse B cells is associated with increased T cell costimulation and the development of insulitis. J Immunol 174:680–687
Marzio R, Mauel J, Betz-Corradin S (1999) CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol 21:565–582
Silveira PA, Grey ST (2006) B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab 17:128–135
Hastings WD, Tumang JR, Behrens TW, Rothstein TL (2006) Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur J Immunol 36:1114–1123
Ghosn EE, Yang Y, Tung J, Herzenberg LA, Herzenberg LA (2008) CD11b expression distinguishes sequential stages of peritoneal B-1 development. Proc Natl Acad Sci U S A 105:5195–5200
Stoermann B, Kretschmer K, Duber S, Weiss S (2007) B-1a cells are imprinted by the microenvironment in spleen and peritoneum. Eur J Immunol 37:1613–1620
Wong FS, Wen L, Tang M et al (2004) Investigation of the role of B cells in type 1 diabetes in the NOD mouse. Diabetes 53:2581–2587
Noorchashm H, Lieu YK, Noorchashm N et al (1999) I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol 163:743–750
Acknowledgements
This work was supported by The Academy of Finland, The Päivikki and Sakari Sohlberg Foundation, Finland, The Finnish Diabetes Research Foundation, The Danisco Research Foundation and Turku University Foundation, Finland. We thank M. Vainio (Turku University of Applied Sciences) and S. Lindqvist (Central Animal Laboratory of Turku University) for skilful technical assistance.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, C., Valkonen, S., Ohls, S. et al. Enhanced trafficking to the pancreatic lymph nodes and auto-antigen presentation capacity distinguishes peritoneal B lymphocytes in non-obese diabetic mice. Diabetologia 53, 346–355 (2010). https://doi.org/10.1007/s00125-009-1599-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1599-x