Abstract
Aims/hypothesis
Children with high-risk type 1 diabetes HLA genotype have increased risk of high relative birthweight (HrBW), while cord blood islet autoantibodies decrease the risk. As gestational infections may affect offspring type 1 diabetes risk, the aims were to test whether: (1) children of mothers reporting gestational infections have increased HrBW; (2) gestational infections explain islet autoantibody reduction of HrBW; and (3) gestational infections affect the association between HLA and HrBW.
Subjects and methods
HLA genotypes and autoantibodies to glutamic acid decarboxylase, insulinoma-associated protein 2 and insulin were determined in cord blood of children born to non-diabetic mothers in the Diabetes Prediction in Skåne (DiPiS) study. Mothers reported gestational infections when the child was 2 months old.
Results
Fever or gastroenteritis during pregnancy was reported by 2,848/19,756 mothers (14%); 339 in more than one trimester. Children whose mothers reported infections had increased risk of HrBW (p = 0.0003), particularly in the absence of cord blood islet autoantibodies (interaction between HrBW, islet autoantibodies and infections, p = 0.0005). The effect on HrBW by high-risk HLA-DQ2/8 was aggravated by infections in more than one trimester (odds ratio [OR] = 5.24; p = 0.003) (interaction; p = 0.022). When infections were reported, cord blood islet autoantibodies decreased HrBW (OR = 0.34; p = 0.0002).
Conclusions/interpretation
This study revealed that: (1) gestational fever, gastroenteritis, or both, increased the risk of HrBW; (2) cord blood islet autoantibodies decreased the risk of HrBW only in combination with infections; and (3) infections aggravated the association between HLA-DQ2/8 and HrBW. These data suggest an interaction between HLA, gestational infections, islet autoantibodies and fetal growth.
Similar content being viewed by others
Introduction
Type 1 diabetes is a chronic autoimmune disease associated with destruction of the pancreatic islet beta cells, resulting in loss of insulin production. The incidence of type 1 diabetes before 18 years of age is increasing [1, 2], and children tend to be diagnosed at an early age [2–5]. HLA genes are strong susceptibility factors for type 1 diabetes [6] but twin studies suggest that environmental factors are also of importance [7, 8]. The low frequency of first-degree relatives (10–15%) with diabetes among children newly diagnosed with type 1 diabetes further supports the importance of environmental factors. High birthweight (BW) has been reported as a risk factor for type 1 diabetes [9–11], in contrast to low BW for gestational age (small for gestational age), which is a risk factor for type 2 diabetes [12, 13]. Among environmental factors, viral infections have frequently been proposed as triggers of the autoimmune reaction resulting in type 1 diabetes. In several studies, it was shown that children who developed type 1 diabetes were more often born to mothers with Coxsackie or echovirus infections compared with control children [14–16], although this could not be confirmed in another study [17]. Children with congenital rubella have been reported to have a high frequency of diabetes later in life [18, 19]. Infections with Coxsackie B virus were reported to occur at an increased frequency in children with newly diagnosed type 1 diabetes compared with control children [20–22], but convincing evidence for or against an association between Coxsackie B virus infection and type 1 diabetes has not been provided [23].
The autoimmune process destroying the beta cells is marked by autoantibodies to glutamic acid decarboxylase (GAD65Ab) [24], insulinoma-associated protein 2 (IA-2Ab) [25, 26] or insulin (IAA) [27]. Up to 90% of children with newly diagnosed type 1 diabetes have at least one of these antibodies. In children born to non-diabetic mothers, autoantibodies in cord blood have retrospectively been found in some children who later developed type 1 diabetes [28]. In contrast, a recent study of children born to mothers with type 1 diabetes reported that GAD65Ab and IA-2Ab in cord blood reduced the risk of developing multiple autoantibodies, diabetes, or both, later in childhood [29].
Recently we reported that children with HLA conferring a risk of type 1 diabetes had an increased frequency of a high relative BW (rBW: BW corrected for gestational age) [30], suggesting that this high relative BW (HrBW) as a possible risk factor for type 1 diabetes may be affected by HLA. In our study, children born to mothers with diabetes were excluded [30]. It is of interest therefore that an association between HLA and BW was also reported in children born to mothers or fathers with type 1 diabetes [31]. We speculated that this effect by HLA on intrauterine growth might also be influenced or mediated by other reported risk factors for type 1 diabetes. For example, we also reported that rBW was reduced in children born to non-diabetic mothers with GAD65Ab [30]. Since both islet autoantibodies [28] and gestational infections [15, 16] have been proposed as risk factors for type 1 diabetes, the aim of the present study was to test the following hypotheses: (1) children born to mothers reporting gestational infections have an increased risk of HrBW; (2) gestational infections explain that islet autoantibodies are reducing the risk of HrBW; and finally (3) that gestational infections affect the association between HLA and HrBW. The hypotheses were tested in an extended cohort (19,756 children) of the previously described Diabetes Prediction in Skåne (DiPiS) study [30, 32].
Subjects and methods
Population
The children in this study participated in DiPiS, a prospective population-based study in Skåne, the most southern province of Sweden with 1.2 million inhabitants [30, 32, 33]. The aim of DiPiS is to determine the positive predictive value for type 1 diabetes of genetic risk combined with islet cell autoantibody markers and to identify factors during and after pregnancy that may trigger type 1 diabetes.
Cord blood was genotyped for HLA and analysed for GAD65Ab, IA-2Ab and IAA. The parents filled out the consent form and a questionnaire (partly described in Electronic supplementary material [ESM]) when the child was 2 months of age. Apart from family history of diabetes, BW and gestational age, the questionnaire allowed the mothers to report infections during pregnancy. There were two questions on infections including infectious diarrhoea, vomiting and febrile illness (above 38°C), frequency of illness and during which trimester (see ESM). The Lund University Ethics Committee approved the DiPiS study.
Study group
Children included in the DiPiS study between September 2000 and August 2004 were analysed. During this period, 48,058 children were born in the province of Skåne (Fig. 1) and 35,683 cord blood samples were obtained at the five maternity clinics in Malmö, Lund, Helsingborg, Kristianstad and Ystad. After excluding twins, triplets, preterm children (born before 37 weeks of gestation) and children born to mothers with diabetes, 31,446 children remained. BW, gestational age and HLA were available from 19,756 children. A limited number of mothers had more than one delivery within the inclusion period of the study. The study design precluded an identification of these siblings. We compared participating with non-participating children, to reveal that participation was higher with increasing age of the mother and with increasing gestational age of the child (ESM Table 1). The participation was also slightly higher when the child had high-risk diabetes HLA genotype, but there was no difference if the child had islet autoantibodies in the cord blood or not (ESM Table 1).
HLA genotyping
HLA haplotypes were determined as previously described [30, 34, 35]. Briefly, cord blood was dropped onto Whatman filters to generate dried blood spots (DBS). The DBS was used to obtain 3-mm punches, which were used directly for PCR amplification of DQA1 and DQB1 alleles as described [30]. Single-stranded DNA was hybridised with two sets of probes, the first containing Eu-DQB1*0602/3, Sm-DQB1*0603/4 and Tb-Control and the second containing Eu-DQB1*0302, Sm-DQB1*0301 and Tb-DQB1*02. Samples positive for DQB1*02 were further analysed for DQA1*0201 and 05 alleles to separate subjects with DR3 from DR7. HLA-DQA1 typing was performed with the same technique as for DQB1 typing with some modifications as described [30] with use of Sm-DQA1*05 and Tb-DQA1*0201 probes.
The HLA genotypes were divided into seven risk groups for type 1 diabetes: (1) very high risk, having DQB1*0201-A1*0501 and DQB1*0302-A1*0301 (DQ2/8) (n = 746); (2) high risk, having DQB1*0302-A1*0301 (DQ8) but not DQB1*0201-A1*0501 (DQ2) (n = 1,860); (3) moderate risk, having DQB1*0201-DQA1*0501 (DQ2) but not DQB1*0302-A1*0301 (DQ8) (n = 1,682); (4) neutral HLA (n = 5,188); (5) low-risk HLA (n = 2,373); (6) very-low-risk (n = 3,087); and (7) no-risk HLA (n = 4,820), as described previously [30].
Autoantibodies to GAD65, IA-2 and insulin
Autoantibodies to GAD65 and IA-2 were determined in DBSs as previously described [30, 36]. In brief, DBS eluates were incubated with labelled antigen and immune complexes precipitated using Protein A-Sepharose. The radioactivity in the precipitate was measured and positive samples (combined GAD65Ab and IA-2Ab analysis >95th percentile) were re-analysed for GAD65Ab and IA-2Ab in separate assays [37, 38]. IAA were analysed with a recently described microassay, which was adapted for a microtitre plate format [39].
Statistical methods and dataset
All questionnaires and forms from the DiPiS-study were scanned into a database, BC/OS system, Biocomputing OS 2000 (Biocomputing Platforms Ltd Oy, Espoo, Finland). In term newborns, rBW was calculated as previously described [30]. Briefly, z scores for each gestational week and sex was calculated by the following formula: z score = (BW−mean [BW])/SD [BW] and termed as rBW.
Population means (SD) of BW were estimated internally using the study group and relative BW was divided into quartiles. Children in the lower quartile were defined as having a low rBW (LrBW) and children in the upper quartile had HrBW. Differences in proportions were tested using chi-square tests. The linear association of HrBW with number of infections within HLA risk groups was tested using a χ 2 test for trend. Interactions between cord blood autoantibodies and infections on the risk of HrBW were tested using logistic regression models. Logistic regression models also were used to examine whether HLA and cord blood autoantibodies were independently associated with HrBW after adjusting for other confounding factors such as sex, gestational age, jaundice, presence of siblings, health of the newborn, presence of jaundice, smoking during pregnancy, maternal age, education of the mother, mother working during pregnancy, lengthy or serious disease in the mother over the previous 5 years and maternal weight gain. p values less than 0.05 were considered significant.
Results
Are infections during pregnancy related to BW?
Of the 19,756 mothers answering the questionnaire, 14.4% reported fever, gastroenteritis or both (diarrhoea, vomiting or both) during pregnancy (ESM Table 2). Fever was more frequently reported (10.7%) than gastroenteritis (7.7%), while 3.0% of the mothers reported both in the same trimester. In total, 1.7% of the 19,756 mothers reported fever, gastroenteritis, or both, in more than one trimester (ESM Table 2). The mothers tended to report more infections if they were older (>30 years), better educated, had a previous child or reported a serious or lengthy disease in the last 5 years (Table 1). There was also a weak association between smoking and fewer reported infections, but this was entirely explained by lower education in smoking mothers. Cord blood autoantibodies were not associated with the number of infections reported. However, infections were associated with increased risk of HrBW (p = 0.0003) (Table 1). There was no difference in odds ratio (OR) between the effect of fever (OR = 1.17 [95% CI 1.06–1.30], p = 0.002) or gastroenteritis (OR = 1.13 [95% CI 0.99–1.28], p = 0.07) on HrBW, but the frequency of infections was of importance (Table 1). When examining the results separately by each trimester, HrBW was associated with infections in the first trimester (OR = 1.22 [95% CI 1.06–1.40], p = 0.005) and second trimester (OR = 1.21 [95% CI 1.06–1.38], p = 0.006) but not with infections during the third trimester (OR = 1.11 [95% CI 0.97–1.28], p = 0.14).
Are autoantibodies and infections related to rBW?
Among all newborns, cord blood GAD65Ab was associated with decreased risk of HrBW (OR = 0.82 [95% CI 0.67–0.99], p = 0.045) as previously described [30], while there was no effect of IA-2Ab or IAA. However, when infections during pregnancy were reported, both GAD65Ab (OR = 0.35 [95% CI 0.19–0.68], p = 0.002) and IAA (OR = 0.21 [95% CI 0.06–0.80], p = 0.02) were significantly associated with decreased risk of HrBW, as well as several autoantibodies (GAD65Ab, IA-2Ab and IAA) (OR = 0.34 [95% CI 0.19–0.59], p = 0.0002) (Table 2). There was no association between islet autoantibodies and HrBW in children of mothers without reported infections.
Are type 1 diabetes-risk HLA genotypes and infections during pregnancy related to rBW?
No difference was found in the frequencies of infection with different HLA genotypes. In children born with HLA-DQ2/8 and without cord blood autoantibodies, there was a linear trend with increasing frequency of children with HrBW among mothers reporting more infections (p = 0.007) (Table 3).
When adjusting for confounding factors by multiple regression analysis, the significant association observed between HrBW, HLA and infections was confirmed, and the negative association between HrBW and islet autoantibodies in the presence of infection remained pronounced (Table 4).
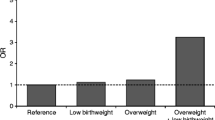
In addition, the association between HLA-DQ2/8 and HrBW was aggravated when mothers reported infections in more than one trimester (Table 4) (interaction between HLA, HrBW and infections; p = 0.022). Nevertheless, although the association between HLA and HrBW when infections were reported in more than one trimester was strongly positive (OR = 5.24 [95% CI 1.75–15.7], p = 0.003), there was still a weak but significant association when no infections were reported (1.22 [95% CI 1.01–1.48], p = 0.04). Thus, infections with fever, gastroenteritis, or both, during pregnancy influenced, but did not explain all, the association between HLA-DQ2/8 and HrBW (Table 4). The non-diabetes risk-related DQB1*0603 was also associated with HrBW (p = 0.02), but only when several infections were reported (Table 4).
Discussion
In this study we further analysed our previously reported associations between HrBW and high-risk diabetes HLA genotypes, as well as islet autoantibodies, by testing the hypothesis that infections during pregnancy may affect the rBW [30]. The major observation in the present investigation is that there was a significant, but complex interaction between rBW and infections and islet autoantibodies as well as HLA.
First, reported infections with fever, gastroenteritis, or both, increased the risk of HrBW. This finding supported our hypothesis that reported gestational infections in the general population of non-diabetic mothers might increase the risk of the child being born with HrBW. This type of association in humans has not, to the best of our knowledge, been reported before, although an adipogenic effect of adenovirus has been described [40].
Second, the significant decreased risk of HrBW in the presence of cord blood islet autoantibodies [30] was observed only when the mother reported fever, gastroenteritis, or both, during pregnancy. Thus, our previously reported reduced risk of HrBW in the presence of GAD65Ab may be explained by gestational infections. We therefore considered the possibility that infections during pregnancy would induce islet autoantibodies. However, our data did not reveal an increased incidence of positive cord blood autoantibodies in children born to mothers who reported gestational infections.
The third major finding was that reported infections during pregnancy aggravated, but did not explain all of the previously reported association between HLA and HrBW [30] (Table 4). The remarkable increase in the risk of HrBW in HLA-DQ2/8-positive children born to mothers reporting infections is of considerable interest as more than 30% of children developing type 1 diabetes have this HLA genotype [41, 42].
Taken together, our major finding was that infections interact with HLA in modifying intrauterine growth, resulting in children with HrBW. This finding is supported by our observation that also the diabetes low-risk allele, DQB1*0603, had an increased risk of HrBW when fever, gastroenteritis, or both, was reported in more than one trimester. Thus, it cannot be excluded that infections during pregnancy may explain the previously reported increase in BW with the DQB1*0603-linked DR13 [43].
At present there is no simple explanation of the interaction between infections, HLA of the child and HrBW. Our speculation is that certain infectious agents are taken up by antigen-presenting cells in the mother, processed by these cells and then presented at the cell surface by HLA class heterodimeric proteins as part of the trimolecular complex. The peptides presented by HLA will be different for different HLA genotypes but may nevertheless induce a vigorous immune response when seen by the T cell receptors on CD4-positive T lymphocytes. The ensuing immune response is expected to induce a marked production in cytokines, resulting in fever and metabolic changes. It is, for example, possible to speculate that the well-known insulin resistance during infection may lead to hyperinsulinaemia, resulting in an increased growth of the fetus. Alternatively, transient hyperglycaemia associated with insulin resistance might also affect fetal growth. While metabolic responses in the mother are likely to influence the growth of the fetus, other explanations of the interaction between infections, HLA and HrBW may include a direct effect of the infectious agent on the fetus.
In the present study, we HLA typed the child, not the mother, but at least one of the HLA heterodimers of the fetus will be shared with the mother. It is therefore possible that the effect of an infection is aggravated if the mother and the child share an HLA genotype that is associated with a vigorous immune response to a certain infectious agent. Further studies will be needed to dissect these intricate but potentially important interactions between infections and fetal growth.
The present study is a unique population-based cohort of newborn children representing 35,683/48,058 newborns during 4 years of ascertainment. Although it was not possible to obtain complete questionnaires from all the parents to be filled out when the child was 2 months of age, our previous analyses indicate that there was little bias in missing data [30, 33]. Since the study design was population-based in a defined geographical area and since the mothers answered the questionnaires without any information about HLA risk of the child or cord blood autoantibodies, the risk of recall bias was minimised. This study therefore allowed us to examine differences in rBW, to reveal relationships between rBW and reported infections, and HLA as well as cord blood autoantibodies. When excluding children born to diabetic mothers, preterm babies and twins or triplets, we removed potential confounders without reducing statistical power.
The questionnaire response rate of 67% might have given some bias to different subgroups as those displayed in ESM Table 1. Although our results reveal that infections affect rBW, it is important to note that HLA genotypes are influencing intrauterine growth also in the absence of reported infections. This observation may be a result of under-reporting of infections due to the fact that mothers do not recall minor infectious events, especially if they occurred early in pregnancy. The optimal solution to this problem would have been blood sampling during pregnancy, but this could not be done in this study. The frequency of reported gastroenteritis was low in our study (6.7%) compared with a recent study (30%), where no relationship was seen between BW and gastroenteritis [44]. However, the author did report a small increase in preterm labour in women with gastroenteritis in months 4, 5 and 7 of pregnancy [44]. The difference in frequencies of gastroenteritis between these two studies may be due to imprecise reporting of infections. Another possible explanation could be that our question to the mothers was distinctly focused on infectious gastroenteritis and fever, and did not include chronic diarrhoea or nausea during early pregnancy (ESM: Questionnaire, Question 1). Further studies will be needed to clarify frequencies and type of infections in relation to rBW and the HLA of both the mother and the child.
BW in the general Swedish population of newborns is increasing, especially the incidence of children with a high BW and those large for gestational age [45]. BW corrected for gestational age, rBW, is an outcome of intrauterine growth rate, which is influenced by multiple factors such as intrauterine infections, cord blood flow, maternal disease, drugs and maternal weight gain [46, 47] as well as the HLA type of the child [30]. High BW and increased growth in infancy have been reported as risk factors for type 1 diabetes [9–11]. This phenomenon is not understood. Prior studies by others have not examined relationships between rBW and HLA as well as islet autoantibodies in the newborn child. Our data suggest that the interaction between HLA, islet autoantibodies and rBW is complex. We interpret the data in Table 4 to indicate that islet autoantibodies in the cord blood of children of mothers reporting infections reduced the rBW regardless of the HLA of the child. Although we do not know the HLA type of the mother we would anticipate that she may carry at least one allele that is diabetes high-risk, which in turn is associated with islet autoantibodies also in the healthy population [41]. It is therefore possible that the reduced risk of HrBW is due to the possibility that islet autoimmunity was already present in some mothers reporting infections. The immune response to infections may be affected in islet autoantibody-positive mothers in a way that fetal growth is reduced rather than accelerated by a gestational infection.
Thus, the previously reported association between high BW and risk of type 1 diabetes [9], may be explained by the interaction between infections and diabetes high-risk HLA affecting intrauterine growth. This is, to the best of our knowledge, a novel finding and may be important for the understanding of type 1 diabetes aetiology.
In conclusion, our reported [30] association between diabetes high-risk HLA and HrBW may be explained by infections during pregnancy. The aggravating effect on HrBW by infections also on HLA-DQB1*0603 (DR13) is underscoring the importance of HLA. While mothers reporting infections had no increased risk to give birth to a child with cord blood islet autoantibodies, their islet autoimmunity was associated with a reduced risk of HrBW. Gestational islet autoimmunity could therefore possibly explain the observation that children born with islet autoantibodies have a reduced risk of developing autoantibodies against GAD65, IA-2 or insulin later in life [29]. As reported infections affected rBW in association with HLA, it will be necessary in future studies to determine the nature of such infections and carefully document the infectious agents. The children affected by intrauterine infections may have an altered infant growth rate, which may be important for type 1 diabetes risk [48–50]. The DiPiS study is longitudinal and children in this report are followed for the development of islet autoimmunity and type 1 diabetes as well as growth. These future analyses may allow us to establish whether the complex interaction between rBW and infections, and HLA as well as islet autoantibodies is increasing the risk of type 1 diabetes.
Abbreviations
- BW:
-
birthweight
- DBS:
-
dried blood spot
- DiPiS:
-
Diabetes Prediction in Skåne
- GAD65Ab:
-
autoantibodies to glutamic acid decarboxylase
- HrBW:
-
high relative birthweight
- IAA:
-
insulin autoantibodies
- IA-2Ab:
-
autoantibodies to insulinoma-associated protein 2
- LrBW:
-
low relative birthweight
- OR:
-
odds ratio
- rBW:
-
relative birthweight
References
Green A, Patterson CC on behalf of the EURODIAB TIGER Study Group (2001) Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia 44(Suppl 3):B3–B8
DIAMOND Project Group (2006) Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med 23:857–866
Dahlquist G, Mustonen L (1994) Childhood onset diabetes-time trends and climatological factors. Int J Epidemiol 23:1234–1241
Gardner SG, Bingley PJ, Sawtell PA, Weeks S, Gale EA (1997) Rising incidence of insulin dependent diabetes in children aged under 5 years in the Oxford region: time trend analysis. The Bart’s-Oxford Study Group. BMJ 315:713–717
Karvonen M, Pitkaniemi J, Tuomilehto J (1999) The onset age of type 1 diabetes in Finnish children has become younger. The Finnish Childhood Diabetes Registry Group. Diabetes Care 22:1066–1070
Redondo MJ, Fain PR, Eisenbarth GS (2001) Genetics of type 1A diabetes. Recent Prog Horm Res 56:69–89
Barnett AH, Eff C, Leslie RD, Pyke DA (1981) Diabetes in identical twins. A study of 200 pairs. Diabetologia 20:87–93
Kaprio J, Tuomilehto J, Koskenvuo M et al (1992) Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia 35:1060–1067
Dahlquist G, Bennich SS, Kallen B (1996) Intrauterine growth pattern and risk of childhood onset insulin dependent (type I) diabetes: population based case-control study. BMJ 313:1174–1177
Stene LC, Magnus P, Lie RT, Sovik O, Joner G (2001) Birth weight and childhood onset type 1 diabetes: population based cohort study. BMJ 322:889–892
Cardwell CR, Carson DJ, Patterson CC (2005) Parental age at delivery, birth order, birth weight and gestational age are associated with the risk of childhood type 1 diabetes: a UK regional retrospective cohort study. Diabet Med 22:200–206
Levy-Marchal C, Jaquet D (2004) Long-term metabolic consequences of being born small for gestational age. Pediatr Diabetes 5:147–153
Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA (2002) Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab 87:4657–4661
Dahlquist G, Frisk G, Ivarsson SA, Svanberg L, Forsgren M, Diderholm H (1995) Indications that maternal Coxsackie B virus infection during pregnancy is a risk factor for childhood-onset IDDM. Diabetologia 38:1371–1373
Dahlquist GG, Ivarsson S, Lindberg B, Forsgren M (1995) Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM. A population-based case-control study. Diabetes 44:408–413
Hyoty H, Hiltunen M, Knip M et al (1995) A prospective study of the role of Coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes 44:652–657
Viskari HR, Roivainen M, Reunanen A et al (2002) Maternal first-trimester enterovirus infection and future risk of type 1 diabetes in the exposed fetus. Diabetes 51:2568–2571
Forrest JM, Menser MA, Burgess JA (1971) High frequency of diabetes mellitus in young adults with congenital rubella. Lancet 2:332–334
Menser MA, Forrest JM, Bransby RD (1978) Rubella infection and diabetes mellitus. Lancet 1:57–60
Ray CG, Palmer JP, Crossley JR, Williams RH (1980) Coxsackie B virus antibody responses in juvenile-onset diabetes mellitus. Clin Endocrinol (Oxf) 12:375–378
Banatvala JE, Bryant J, Schernthaner G et al (1985) Coxsackie B, mumps, rubella, and cytomegalovirus specific IgM responses in patients with juvenile-onset insulin-dependent diabetes mellitus in Britain, Austria, and Australia. Lancet 1:1409–1412
Hyoty H, Taylor KW (2002) The role of viruses in human diabetes. Diabetologia 45:1353–1361
Green J, Casabonne D, Newton R (2004) Coxsackie B virus serology and type 1 diabetes mellitus: a systematic review of published case-control studies. Diabet Med 21:507–514
Baekkeskov S, Aanstoot HJ, Christgau S et al (1990) Identification of the 64 K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347:151–156
Solimena M, Dirkx R Jr, Hermel JM et al (1996) ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J 15:2102–2114
Saeki K, Zhu M, Kubosaki A, Xie J, Lan MS, Notkins AL (2002) Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes 51:1842–1850
Atkinson MA, Eisenbarth GS (2001) Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358:221–229
Lindberg B, Ivarsson SA, Landin-Olsson M, Sundkvist G, Svanberg L, Lernmark A (1999) Islet autoantibodies in cord blood from children who developed type I (insulin-dependent) diabetes mellitus before 15 years of age. Diabetologia 42:181–187
Koczwara K, Bonifacio E, Ziegler AG (2004) Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes 53:1–4
Larsson HE, Lynch K, Lernmark B et al (2005) Diabetes-associated HLA genotypes affect birthweight in the general population. Diabetologia 48:1484–1491
Hummel M, Marienffeld S, Huppmann M et al (2006) Interaction between maternal type 1 diabetes and HLA DR4 increases fetal growth. Diabetologia 49(Suppl 1):68
Larsson K, Elding-Larsson H, Cederwall E et al (2004) Genetic and perinatal factors as risk for childhood type 1 diabetes. Diabetes Metab Res Rev 20:429–437
Lernmark B, Elding-Larsson H, Hansson G, Lindberg B, Lynch K, Sjoblad S (2004) Parent responses to participation in genetic screening for diabetes risk. Pediatr Diabetes 5:174–181
Ilonen J, Reijonen H, Herva E et al (1996) Rapid HLA-DQB1 genotyping for four alleles in the assessment of risk for IDDM in the Finnish population. The Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care 19:795–800
Sjoroos M, Iitia A, Ilonen J, Reijonen H, Lovgren T (1995) Triple-label hybridization assay for type-1 diabetes-related HLA alleles. Biotechniques 18:870–877
Hampe CS, Hammerle LP, Bekris L et al (2000) Recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different GAD antibody-positive phenotypes. J Clin Endocrinol Metab 85:4671–4679
Grubin CE, Daniels T, Toivola B et al (1994) A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia 37:344–350
Verge CF, Stenger D, Bonifacio E et al (1998) Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: combinatorial islet autoantibody workshop. Diabetes 47:1857–1866
Williams AJ, Bingley PJ, Bonifacio E, Palmer JP, Gale EA (1997) A novel micro-assay for insulin autoantibodies. J Autoimmun 10:473–478
Atkinson RL, Dhurandhar NV, Allison DB et al (2005) Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond) 29:281–286
Graham J, Hagopian WA, Kockum I et al (2002) Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 51:1346–1355
Lambert AP, Gillespie KM, Thomson G et al (2004) Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J Clin Endocrinol Metab 89:4037–4043
Aroviita P, Partanen J, Sistonen P, Teramo K, Kekomaki R (2004) High birth weight is associated with human leukocyte antigen (HLA) DRB1*13 in full-term infants. Eur J Immunogenet 31:21–26
Ludvigsson JF (2001) Effect of gastroenteritis during pregnancy on neonatal outcome. Eur J Clin Microbiol Infect Dis 20:843–849
National Board of Health and Welfare (2002) Fakta om mammor, förlossningar, nyfödda barn-medicinska födelseregistret 1973–2000. Medical birth registry
Brooke OG, Anderson HR, Bland JM, Peacock JL, Stewart CM (1989) Effects on birth weight of smoking, alcohol, caffeine, socioeconomic factors, and psychosocial stress. BMJ 298:795–801
Hutcheon JA, Platt RW, Meltzer SJ, Egeland GM (2006) Is birth weight modified during pregnancy? Using sibling differences to understand the impact of blood glucose, obesity, and maternal weight gain in gestational diabetes. Am J Obstet Gynecol 195:488–494
Blom L, Persson LA, Dahlquist G (1992) A high linear growth is associated with an increased risk of childhood diabetes mellitus. Diabetologia 35:528–533
EURODIAB Substudy 2 Study Group (2002) Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 25:1755–1760
Hypponen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK (2000) Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care 23:1755–1760
Acknowledgements
Co-authors in the DiPiS study group are: P. Almgren, B. Buveris-Svendburg, A. Carlsson, E. Cederwall, C. Cilio, J. Gerardsson, B. Jönsson, K. Kockum, K. Larsson, B. Lindberg, J. Neiderud, A. Nilsson, M. Pelkonen, H. Rastkhani and S. Sjöblad. We thank all the participating parents and children in DiPiS. Our research is supported in part by the Swedish Research Council (Grant 14064), Juvenile Diabetes Research Foundation, Wallenberg Foundation, Swedish Childhood Diabetes Foundation, Swedish Diabetes Association, Nordisk Insulin Fund, National Institutes of Health (DK26190), UMAS funds, Terry & Louise Gregg Diabetes in Pregnancy Award from the American Diabetes Association, Lion Club International, District 101-S and the Skåne County Council Foundation for Research and Development.
Duality of interest
There is no duality of interest
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Members of the DiPiS Study Group are listed in the Acknowledgements
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Larsson, H.E., Lynch, K., Lernmark, B. et al. Relationship between increased relative birthweight and infections during pregnancy in children with a high-risk diabetes HLA genotype. Diabetologia 50, 1161–1169 (2007). https://doi.org/10.1007/s00125-007-0648-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0648-6