Abstract
Aims/hypothesis
Cinnamon extracts have anti-diabetic effects. Phenolic acids, including hydrocinnamic acids, were identified as major components of cinnamon extracts. Against this background we sought to develop a new anti-diabetic compound using derivatives of hydroxycinnamic acids purified from cinnamon.
Methods
We purified hydroxycinnamic acids from cinnamon, synthesised a series of derivatives, and screened them for glucose transport activity in vitro. We then selected the compound with the highest glucose transport activity in epididymal adipocytes isolated from male Sprague–Dawley rats in vitro, tested it for glucose-lowering activity in vivo, and studied the mechanisms involved.
Results
A naphthalenemethyl ester of 3,4-dihydroxyhydrocinnamic acid (DHH105) showed the highest glucose transport activity in vitro. Treatment of streptozotocin-induced diabetic C57BL/6 mice and spontaneously diabetic ob/ob mice with DHH105 decreased blood glucose levels to near normoglycaemia. Further studies revealed that DHH105 increased the maximum speed of glucose transport and the translocation of glucose transporter 4 (GLUT4, now known as solute carrier family 2 [facilitated glucose transporter], member 4 [SLC2A4]) in adipocytes, resulting in increased glucose uptake. In addition, DHH105 enhanced phosphorylation of the insulin receptor-β subunit and insulin receptor substrate-1 in adipocytes, both in vitro and in vivo. This resulted in the activation of phosphatidylinositol 3-kinase and Akt/protein kinase B, contributing to the translocation of GLUT4 to the plasma membrane.
Conclusions/interpretation
We conclude that DHH105 lowers blood glucose levels through the enhancement of glucose transport, mediated by an increase in insulin-receptor signalling. DHH105 may be a valuable candidate for a new anti-diabetic drug.
Similar content being viewed by others
Introduction
Impaired glucose transport is a major factor contributing to insulin resistance in type 2 diabetic patients [1]. The ability of insulin to mediate tissue glucose uptake is critical to maintenance of glucose homeostasis and to the clearing of a post-prandial glucose load [2, 3]. Glucose transport is mediated by specific glucose transporters—in the case of insulin-stimulated glucose transport largely by glucose transporter 4 (GLUT4, now known as solute carrier family 2 [facilitated glucose transporter], member 4 [SLC2A4]) [4]. Binding of insulin to its receptor activates the receptor’s tyrosine kinase activity, which phosphorylates a number of downstream substrates, such as IRS-1 and IRS-2. Phosphorylated IRS-1 and -2 bind to various other substrates and trigger insulin actions, such as glucose uptake [5, 6].
Drugs used for the treatment of type 2 diabetes have various mechanisms of action that target the lesions leading to insulin resistance and beta cell dysfunction [7]. In addition, type 2 diabetes and obesity commonly co-exist, and several agents are in development to improve glycaemic control without increasing body weight [8]. Herbal plants have been used for medicinal purposes for centuries. Cinnamon, widely consumed in Asian countries, is known to have anti-diabetic effects [9–13]. Cinnamon contains flavonoids, phenolic acids including hydroxycinnamic acids, and tannins. The present investigation was initiated to identify the anti-diabetic compound in cinnamon by using derivatives of hydroxycinnamic acids purified from cinnamon. Further studies were conducted on the most effective glucose-lowering derivative, a naphthalenemethyl ester of 3,4-dihydroxyhydrocinnamic acid (DHH105), to find the mechanisms involved in its anti-diabetic effect, particularly its possible effect on glucose transport and insulin receptor (IR) signalling pathways.
Materials and methods
Animals
C57BL/6 and C57BL/6 ob/ob mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and Sprague–Dawley rats were purchased from Charles River Laboratories (Wilmington, MA, USA). Male animals were used in all experiments. The animals were maintained under specific pathogen-free conditions at the Animal Resources Centre, Faculty of Medicine, University of Calgary under a photoperiod of 12-h light/12-h darkness at 22 to 23°C. Free access to sterile food and water was provided. Rats were housed three animals/cage, mice five animals/cage. The use and care of the animals used in this study were approved by the Animal Care Committee, Faculty of Medicine, University of Calgary.
Synthesis of hydroxycinnamic acid derivatives including DHH105
Derivatives of dihydroxycinnamic acids (DHH) were synthesised by esterification of hydroxycinnamic acids with various alkyl halides, arylalkyl halides, or hydroxyalkyl halides using NaHCO3 as a base in N,N-dimethylformamide [14] and their chemical structures were analysed by nuclear magnetic resonance (NMR). DHH105 was prepared by esterification of 3,4-dihydroxyhydrocinnamic acid with 1-(chloromethyl)naphthalene using NaHCO3 and KI in N,N-dimethylformamide. Briefly, 3,4-dihydroxyhydrocinnamic acid (32.9 mmol/l) was dissolved in N,N-dimethylformamide (30 ml), and 1-(chloromethyl)naphthalene (33.9 mmol/l), NaHCO3 (50.0 mmol/l) and KI (36.1 mmol/l) were added successively. The reaction mixture was stirred at 80°C for 3 h, cooled to room temperature, diluted with water (200 ml), and extracted with ethyl acetate (3×100 ml). The extracts were washed with water (200 ml) and brine (3×100 ml), dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (4:1 hexane–EtOAc) to afford 8.2 g (77% yield) of DHH105. Structural analysis data of DHH105: (1) 1H NMR analysis (solvent, CD3OD; resolution, 400 MHz). Data δ (ppm); 7.88 (m, 2H), 7.84 (dd, J=7.2, 2.8 Hz, 1H), 7.51 (m, 2H), 7.43 (d, J=2.0 Hz, 1H), 7.42 (d, J=7.2 Hz, 1H), 6.621 (d, J=2.0 Hz, 1 H), 6.62 (d, J=8.0 Hz, 1 H), 6.45 (dd, 8.0, 2.0 Hz, 1 H), 5.52 (s, 2H), 2.77 (t, J=6.8 Hz, 2H), 2.60 (t, J=6.8 Hz, 2H); (2) 13C NMR analysis (solvent, CD3OD; resolution, 100 MHz). Data δ (ppm); 173.5, 145.1, 143.5, 134.0, 132.2, 131.74, 131.68, 129.0, 128.4, 127.2, 126.4, 125.8, 125.1, 123.4, 119.4, 115.3, 115.2, 64.4, 36.1, 30.3.
Induction of diabetes by streptozotocin
Non-fasted, 8-week-old male C57BL/6 mice were injected i.p. with 150 mg/kg streptozotocin (STZ), which was freshly prepared in cold 50 mmol/l citrate buffer, pH 4.0, immediately prior to the injection. One week after the injection, blood glucose levels were measured, and mice with two consecutive blood glucose measurements >16.7 mmol/l were used for experiments.
Administration of DHH105
DHH105 was dissolved in DMSO and emulsified in olive oil (1:50 v/v). DHH105 (150 mg/kg body weight in 100 μl) was injected i.p. daily into 8-week-old diabetic ob/ob mice (blood glucose level >12.8 mmol/l) and STZ-induced diabetic C57BL/6 mice. Control mice received the same amount of vehicle without DHH105. Blood was obtained from a tail clip, and glucose levels were measured daily with a glucometer (One Touch Ultra, Lifescan, Milpitas, CA, USA) between 09:00 and 10:00 hours immediately before injection of DHH105.
Glucose tolerance tests
Glucose tolerance tests were performed after 2 weeks of treatment with DHH105. Briefly, mice were fasted overnight and injected with glucose (2 g/kg body weight, i.p.). Blood glucose levels were measured at 0, 15, 30, 45, 60, and 120 min after glucose injection [15].
Isolation of epididymal adipocytes
Epididymal adipose tissue was removed from 5-week-old male Sprague–Dawley rats and digested with collagenase type II (1 mg/ml; Sigma Chemical, St. Louis, MO, USA) in 1 ml Krebs–Ringer HEPES (KRH) buffer (131 mmol/l NaCl, 4.7 mmol/l KCl, 2.5 mmol/l CaCl2, 1.25 mmol/l MgCl2, 2.5 mmol/l NaH2PO4, 10 mmol/l HEPES, pH 7.4) supplemented with 1% BSA and 2 mmol/l pyruvate for 1 h at 37°C [16]. After digestion, adipocytes were prepared as described previously [17]. Adipocytes were pooled from three or four animals.
Glucose transport assay
To screen DHH derivatives for glucose transport activity, adipocytes (3–4×105 cells/ml) in KRH buffer were treated with various DHH derivatives (200 μmol/l) for 4 h. To examine the dose- and time-dependent effects of DHH105 on glucose transport, adipocytes were treated with various concentrations of DHH105 (0, 25, 50, 100, 250, 500, or 1000 μmol/l) for 4 h or with 500 μmol/l DHH105 for various times (1, 2, 3, or 4 h). To compare the effect of DHH105 on glucose transport with that of insulin, adipocytes were treated with DHH105 (500 μmol/l) for 4 h or insulin (25 ng/ml) for 30 min. To determine whether GLUT4 is involved in glucose transport, phenylarsine oxide (PAO; 1 μmol/l; Sigma) was added 10 min prior to the addition of DHH105 (500 μmol/l). To examine whether phosphatidylinositol 3-kinase (PI3-kinase) is involved in glucose transport, the PI3-kinase inhibitor, LY294002 (50 μmol/l; Sigma) or wortmannin (100 nmol/l; Sigma) was added 10 min prior to the addition of DHH105 (500 μmol/l), and cells were incubated for 4 h. After adipocytes were subjected to the various treatments, 3H-2-deoxyglucose (DOG; 18.5 kBq, 0.125 mmol/l; Amersham Pharmacia Biotech, Buckinghamshire, UK) was added and the cells were incubated for 3 min. Adipocytes were harvested by silicon oil (Sigma) and the radioactivity was measured [18]. Non-specific binding of 3H-2-DOG was measured in the presence of 1.2 mmol/l phloretin (Sigma) [17]. To determine the kinetics of glucose transport, various concentrations of 2-DOG (0.125 to 10.0 mmol/l) were used. The data were fitted to the Michaelis–Menten equation and then transformed with the Lineweaver–Burke equation [19].
Insulin binding competition assay
Isolated adipocytes were incubated in KRH buffer for 1 h. After washing, adipocytes (2×105 cells/vial) in KRH buffer were incubated with DHH105 and/or insulin in the presence of 125I-insulin (0.1 ng/ml; Perkin-Elmer Life and Analytical Sciences, Downers Grove, IL, USA) [20] for 2 h at 16°C in a shaking bath. Non-specific binding was measured in the presence of unlabelled insulin (50 μg, Sigma) and 125I-insulin. After incubation, adipocytes were harvested by silicon oil and the radioactivity was measured [21].
Glycogen synthesis assay
Isolated rat adipocytes (5×105/ml KRH buffer) were incubated for 4 h with DHH105 (500 μmol/l) or 2 h with insulin (100 nmol/l) in 12-well plates in the presence of 11.1 kBq d-[U14C] glucose. After washing in cold PBS, cells were lysed with 300 μl 1 mol/l KOH and transferred to 2-ml microtubes. Carrier glycogen (2.5 mg/ml) was added, and samples were boiled for 5 min. Na2SO4 (saturated; 90 μl) and 1.2 ml cold ethanol (−20°C) were added to each tube, and samples were precipitated overnight at −20°C. After centrifugation, pellets were dissolved in 500 μl water, 1.2 ml ethanol was added, and samples were incubated at −20°C for 20 min. Samples were centrifuged, pellets were dissolved in 500 μl water, and the radioactivity in 400 μl of solution was measured by scintillation counting [22, 23].
Subcellular fractionation of adipocytes
Rat adipocytes treated with DHH105 for 4 h or insulin for 30 min were washed with HEPES, EDTA, sucrose (HES) buffer (8 mmol/l HEPES, 250 mmol/l sucrose, 2 mmol/l EDTA, 20 mmol/l NaF, 50 mmol/l NaVO4, 50 mmol/l sodium pyrophosphate, and protease and phosphatase inhibitor cocktails [Sigma], pH 7.4) and homogenised in 20 ml HES buffer with a potter Elvehjem teflon pestle (15 strokes). Homogenates were centrifuged at 1,000 g for 10 min and the supernatant was centrifuged at 16,000 g for 20 min. The pellet was resuspended in 500 μl HES buffer, carefully laid on a discontinuous sucrose gradient (density top to bottom: 1.0592, 1.1270, 1.1533, 1.1764, 1.2575) and centrifuged at 110,000 g for 70 min. The plasma membrane fraction was obtained from the gradient density of 1.1533, diluted in 50 ml HES buffer, and centrifuged at 16,000 g for 20 min. The pellet (plasma membrane fraction) was resuspended in 500 μl HES buffer and stored at −70°C. To obtain the microsomal membrane fraction, the supernatant from the second centrifugation was centrifuged at 220,000 g for 70 min. The pellet was washed, resuspended in 500 μl HES buffer and stored at −70°C [24]. The protein content of each fraction was measured by the Bradford method [25].
Immunoprecipitation
Isolated rat adipocytes treated with DHH105 for 2 h or insulin for 10 min or epididymal adipose tissue obtained from STZ-induced diabetic C57BL/6 mice or ob/ob mice treated with DHH105 for 2 weeks were homogenised in 5 ml lysis buffer (20 mmol/l Tris, pH 7.4, 1% SDS, 10% glycerol, 150 mmol/l NaCl, 2 mmol/l EDTA, 20 mmol/l NaF, 1 mmol/l NaVO4, 2 mmol/l sodium pyrophosphate, and protease and phosphatase inhibitor cocktails). Part of the lysates was saved for immunoblotting. The remaining lysates (1 μg/μl protein concentration) were incubated with anti-IR-β antibody (Upstate Biotech, Charlottesville, VA, USA) or anti-IRS-1 antibody (Upstate Biotech) overnight at 4°C. Protein A Sepharose beads (100 μl, Amersham Pharmacia Biotech) were added and the mixture was gently rocked for 2 h at 4°C. After centrifugation at full speed in a microcentrifuge for 5 min at 4°C, the pellet was washed and suspended in 2× sample buffer (50 mmol/l Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 0.1% bromophenol blue, 5% β-mercaptoethanol) and boiled.
Immunoblotting
Proteins were separated by 10% SDS-PAGE and transferred to a polyvinyl difluoride membrane (Amersham Pharmacia Biotech). The membranes were incubated with anti-IR-β, anti-IRS-1, or anti-phosphotyrosine (Upstate Biotech); anti-Akt/protein kinase B (Akt/PKB) or anti-phospho-Akt/PKB (Cell Signalling Technology, Beverly, MA, USA); anti-GLUT4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); or anti-β actin (Sigma) antibody in 5% bovine serum albumin in Tris-buffered saline with Tween (TBST; 0.05% Tween 20, 200 mmol/l Tris-HCl, 500 mmol/l NaCl, pH 7.5) for 1 h at room temperature or overnight at 4°C. After washing, the membranes were incubated with secondary antibodies labelled with horseradish peroxidase in 5% skim milk in TBST for 1 h. Immunoreactive proteins were detected with an enhanced chemoluminescence western blot detection kit (Amersham Pharmacia Biotech).
PI3-kinase assay
Phosphatidylinositol in DMSO (1 mg/ml) was mixed with an equal volume of buffer A (10 mmol/l HEPES, 100 mmol/l NaCl, 1 mmol/l EGTA, 0.03% NP-40, pH 7.0) and then 10 μl was mixed with an equal volume of buffer B (500 μmol/l [γ-32P]ATP [370 kBq; Amersham Pharmacia Biotech], 25 mmol/l MgCl2, 15 mmol/l 2-(N-morpholine) ethansulfonic acid, pH 6.8). This mixture was added to 30 μl of adipocyte lysate and incubated for 5 min at room temperature. The kinase reaction was stopped by adding 80 μl of 1 N HCl. A mixture of chloroform and ethanol (1:1, 160 μl) was added to the reaction mixture, followed by centrifugation at full speed in a microcentrifuge for 2 min, after which 40 μl of the organic phase was spotted on silica gel 60 thin layer chromatography plates that were pre-soaked in developing solvent (chloroform–methanol–2.2 mol/l NH4OH, 9:7:2). Samples were run, air-dried, and phosphorylated phosphatidylinositol was detected by autoradiography [26].
Statistical analysis
The significance of differences between groups was analysed by Student’s t test. A level of p<0.05 was accepted as significant.
Results
Identification of DHH105 as an anti-diabetic agent
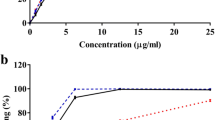
We synthesised a variety of ester derivatives of DHH and determined their structures by nuclear magnetic resonance (Fig. 1a). We then screened these derivatives for glucose transport activity in rat adipocytes. DHH101, DHH103, and DHH105 showed the highest glucose transport activity when tested at a dose of 200 μmol/l and incubation time of 4 h (Fig. 1b). When STZ-induced diabetic C57BL/6 mice were treated with DHH101, DHH103 or DHH105 daily (100 mg/kg, i.p.) for 2 days, only DHH105 significantly decreased blood glucose levels (Fig. 1c); therefore DHH105 was used for further study.
DHH105 treatment decreases blood glucose levels in diabetic mice. a Structures of hydroxycinnamic acids tested. b Isolated rat epididymal adipocytes were treated with dihydroxycinnamic acid derivatives (200 μmol/l) for 4 h. Glucose transport was measured by 3H-DOG uptake and was expressed as percent of basal transport. Data are means±SEM of three independent experiments carried out in triplicate. *p<0.05, **p<0.01 as compared with basal. c STZ-induced diabetic mice were injected with vehicle (white bars), DHH101 (light grey bars), DHH103 (dark grey bars), or DHH105 (100 mg/kg, i.p.; black bars) daily for 2 days, and blood glucose levels were measured. *p<0.05 as compared with vehicle-injected mice, n=5 per group
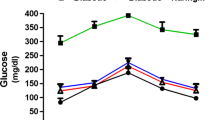
To determine whether DHH105 has an anti-diabetic effect, STZ-induced diabetic C57BL/6 mice were treated with DHH105 daily for 2 weeks. DHH105 treatment significantly lowered blood glucose at 1 and 2 weeks after treatment (Fig. 2a), and glucose clearance after exogenous glucose loading was significantly improved as compared with untreated diabetic control mice (Fig. 2b). When diabetic ob/ob mice were treated with DHH105, blood glucose levels began to decrease after 3 days of DHH105 treatment (Fig. 2c). Although blood glucose levels of all mice fluctuated during the experiment, blood glucose levels of the treated mice remained significantly lower than those of vehicle-treated control mice. Glucose clearance was also significantly improved in DHH105-treated ob/ob mice (Fig. 2d). These results indicate that DHH105 has an anti-diabetic effect in both STZ-induced diabetic C57BL/6 mice and spontaneously diabetic ob/ob mice.
DHH105 treatment decreases blood glucose levels in diabetic mice. STZ-induced diabetic C57BL/6 mice (a, b) or diabetic ob/ob mice (c, d) were injected with vehicle (white bars/circles) or DHH105 (150 mg/kg, i.p.; black bars/circles) daily for 2 weeks. Blood glucose levels were measured (a, c) after the indicated treatment times. b, d After 2 weeks of DHH105 treatment, glucose tolerance tests were performed. Data are means±SEM. *p<0.05, **p<0.01 compared with vehicle-treated, control mice. n=11 (a); n=6 (b); n=5 (c); n=3 (d)
Dose- and time-dependent enhancement of glucose transport in DHH105-treated rat adipocytes
To determine whether DHH105 has a dose- or time-dependent effect on the induction of glucose transport in adipocytes, isolated rat adipocytes were treated with DHH105 at various concentrations and times, and 3H-2-DOG uptake was measured. Uptake of 2-DOG gradually increased in a dose-dependent manner and peaked at a concentration of 500 μmol/l DHH105 (Fig. 3a). Treatment of adipocytes with 500 μmol/l DHH105 resulted in increased 2-DOG uptake after 2 h, with further increases at 3 and 4 h of incubation (Fig. 3b). However, the increase of glucose transport induced by DHH105 was less than that induced by insulin (Fig. 3c).
DHH105 treatment increases glucose transport in isolated rat epididymal adipocytes. Transport of glucose (3H-2-DOG) was measured in a isolated rat epididymal adipocytes treated with the indicated concentrations of DHH105 for 4 h and in b adipocytes treated without (white circles) or with DHH105 (500 μmol/l; black circles) for the indicated times. *p<0.05, **p<0.01 compared with the untreated control group. c Adipocytes were treated with DHH105 (500 μmol/l) for 4 h or insulin (25 ng/ml) for 30 min, and glucose transport was measured. Untreated adipocytes were used as a control. **p<0.01 as compared with control
Increase of GLUT4 translocation in DHH105-treated adipocytes
To determine whether translocation of GLUT4 might be a mechanism by which DHH105 increases glucose uptake in adipocytes, we first examined the kinetics of glucose transport by measuring 2-DOG uptake in the presence of various concentrations of 2-DOG in DHH105-treated adipocytes. The results were plotted according to the Michaelis–Menten equation (Fig. 4a) and transformed by Lineweaver–Burke plot (Fig. 4b). We found that the maximum speed of glucose transport in DHH105-treated adipocytes was threefold higher than in control adipocytes (5.56±0.02 vs 17.14±0.25 mmol/l over a period of 3 min), whereas there was no difference in the Michaelis–Menten constant (K m) value between DHH105-treated and control adipocytes (1.76±0.04 vs 1.78±0.38 mmol/l).
DHH105 increases GLUT4 translocation in adipocytes. Adipocytes were treated without (white circles) or with DHH105 (500 μmol/l; black circles) for 4 h. Glucose transport was measured in the presence of the indicated concentrations of 3H-2-DOG, and the results were plotted using the Michaelis–Menten (a) and Lineweaver–Burke (b) equations. c Adipocytes were treated without or with DHH105 (500 μmol/l) for 4 h in the presence of vehicle (DMSO; white bars) or PAO (1 μmol/l; black bars). Glucose transport was measured by 3H-2-DOG uptake. Data are means±SEM of three independent experiments carried out in triplicate. **p<0.01 as compared with the DHH105-treated group in the absence of PAO. d Isolated rat epididymal adipocytes were treated with 500 μmol/l DHH105 for 4 h or insulin (25 ng/ml) for 30 min. The plasma membrane fraction and microsomal membrane fraction were subjected to SDS-PAGE, labelled with anti-GLUT4 antibody (α-GLUT4), and visualised by horseradish peroxidase-labelled secondary antibody and chemiluminescence. The relative amount of GLUT4 in each fraction (bar graph) was calculated by (GLUT4 intensity in the fraction/GLUT4 intensity in the plasma membrane from untreated control cells [CP]). *p<0.05 as compared with CP, # p<0.05 as compared with CM. A representative gel picture is shown. CP, plasma membrane from untreated control cells; DP, plasma membrane from DHH105-treated cells; IP, plasma membrane from insulin-treated cells; CM, microsomal membrane from untreated control cells; DM, microsomal membrane from DHH105-treated cells; IM, microsomal membrane from insulin-treated cells
To determine the involvement of GLUT4 in the induction of glucose transport by DHH105, we measured 2-DOG uptake in the presence of PAO, which inhibits GLUT4-mediated transport in adipocytes [27, 28]. PAO completely abolished glucose transport induced by DHH105 (Fig. 4c), clearly indicating that GLUT4 is involved in the induction of glucose transport by DHH105 treatment.
Next, we examined whether DHH105 causes translocation of GLUT4 to the plasma membrane. We fractionated cell lysates prepared from DHH105-treated adipocytes and measured the amount of GLUT4 in the plasma and microsomal membrane fractions by western blot using anti-GLUT4 antibody. Immunoreactive GLUT4 in the plasma membrane fraction was increased in DHH105-treated adipocytes as compared with untreated control adipocytes, whereas the amount of GLUT4 in the microsomal membrane fraction was decreased (Fig. 4d). These results indicate that DHH105 induces the translocation of GLUT4 from microsomal membrane to the plasma membrane in adipocytes.
Competitive binding of DHH105 and insulin to adipocytes
To examine whether DHH105 competes with insulin for binding to adipocytes or affects insulin-induced glucose transport, 125I-insulin binding to adipocytes was measured in the presence of DHH105. Addition of DHH105 inhibited the binding of 125I-insulin to adipocytes (Fig. 5a) and addition of DHH105 together with unlabelled insulin further inhibited binding (Fig. 5b). These results indicate that DHH105 may compete with insulin for binding sites on adipocytes. To determine whether DHH105 affects insulin-stimulated glucose transport, glucose transport was examined after treatment with insulin in the presence or absence of DHH105. Insulin treatment increased glucose transport in a dose-dependent manner; however DHH105 treatment did not influence the effect of insulin on glucose transport (Fig. 5c). This result indicates that DHH105 does not inhibit or sensitise the effect of insulin on glucose transport.
DHH105 competes with insulin for binding to adipocytes. a Isolated rat epididymal adipocytes were treated with the indicated concentrations of DHH105 and 125I-insulin (0.1 ng/ml) for 2 h, and insulin binding was measured. b Isolated rat epididymal adipocytes were treated with the indicated concentrations of insulin and 125I-insulin (0.1 ng/ml) in the absence (white circles) or presence (black circles) of 500 μmol/l DHH105 for 2 h, and insulin binding was measured. c Isolated rat adipocytes were treated without (white circles) or with 500 μmol/l DHH105 (black circles) for 3.5 h and the indicated concentrations of insulin were added for 30 min. Glucose transport was measured by 3H-2-DOG uptake
Increased glycogen synthesis by DHH105
Stimulation of adipocytes with insulin induces glucose metabolism, including an increase in glycogen synthesis [29]. To determine whether DHH105 has insulin-like metabolic effects, glycogen synthesis was measured in isolated adipocytes in the presence of DHH105. Treatment of adipocytes with DHH105 increased glycogen synthesis about twofold as compared with untreated adipocytes; this result was similar to insulin, which increased glycogen synthesis about 2.5-fold (Fig. 6).
DHH105 treatment increases glycogen synthesis. Isolated rat adipocytes were treated without (control) or with 500 μmol/l DHH105 for 4 h or insulin (100 nmol/l) for 2 h. Glycogen synthesis was measured and expressed as the fold-increase over the control. Data are means±SEM of three independent experiments carried out in triplicate. **p<0.01 as compared with control
Increased phosphorylation of the insulin receptor β and insulin receptor substrate 1 by DHH105 in vitro and in vivo
To examine whether DHH105 treatment increases autophosphorylation of the IR and its tyrosine kinase activity, the phosphorylation of IR-β and IRS-1 were measured by immunoprecipitation and western blot in rat adipocytes treated with DHH105 in vitro. Phosphorylation of IR-β and IRS-1 was increased in DHH105-treated adipocytes in a similar way to insulin-treated adipocytes, as compared with the untreated control (Fig. 7a,d).
DHH105 treatment increases the phosphorylation of IR-β and IRS-1. a Isolated rat epididymal adipocytes were treated with 500 μmol/l DHH105 (DHH) for 2 h or with 25 ng/ml insulin (INS) for 10 min, and cell lysates were prepared. Con, untreated control. Adipose tissue was removed from STZ-induced diabetic C57BL/6 mice (b) or ob/ob diabetic mice (c) at 2 weeks after treatment with (+) or without (−) DHH105. The phosphorylation of the IR-β subunit or IRS-1 was measured by immunoprecipitation (IP) and western blot (WB) using anti-IR-β (α-IRβ) or anti-IRS-1 antibody (α-IRS-1) and anti-phosphotyrosine (α-PY) antibodies. d–f The band intensities of the phosphorylated proteins from the corresponding blots (a–c) were normalised by dividing each intensity by the band intensity of the relevant non-phosphorylated proteins. These normalised values were used to calculate the relative amounts of phosphorylated proteins as a ratio of treated/control. Results are representative of three independent experiments. White bars, control; black bars, DHH105-treated; grey bars, insulin-treated. **p<0.01 as compared with the control group
To examine whether in vivo treatment of STZ-induced diabetic C57BL/6 mice or ob/ob mice with DHH105 also increases phosphorylation of IR-β and IRS-1, fat tissue was removed from the mice after 2 weeks of DHH105 treatment and phosphorylation of IR-β and IRS-1 was measured by immunoprecipitation and western blot. Consistent with the in vitro results, DHH105 treatment increased phosphorylation of IR-β and IRS-1 in adipose tissue from STZ-induced diabetic C57BL/6 mice (Fig. 7b,e) and ob/ob mice (Fig. 7c,f).
Activation of PI3-kinase and Akt/PKB and its contribution to glucose transport, by treatment with DHH105
To determine whether DHH105 activates PI3-kinase, we examined PI3-kinase activity in DHH105-treated rat adipocytes by measuring the conversion of phosphatidylinositol to phosphatidylinositol 3,4,5-triphosphate (PIP3). The intensity of the PIP3 band was significantly increased in DHH105-treated adipocytes, indicating that DHH105 treatment significantly increased PI3-kinase activity (Fig. 8a,b). To determine whether PI3-kinase is involved in DHH105-induced glucose transport, adipocytes were treated with DHH105 in the presence of PI3-kinase inhibitors, LY294002 or wortmannin, and glucose uptake was measured. Treatment with LY294002 or wortmannin reduced glucose uptake induced by DHH105 to the level of basal glucose uptake (Fig. 8c). These results indicate that the PI3-kinase pathway is involved in DHH105-induced glucose transport in adipocytes. To determine whether DHH105 treatment activates Akt/PKB, the phosphorylation of Akt/PKB was examined in DHH105-treated adipocytes. DHH105 treatment did not change the amount of Akt/PKB protein, but significantly increased the phosphorylated Akt/PKB as compared with the untreated control adipocytes (Fig. 8d). These results indicate that DHH105 can activate Akt/PKB in adipocytes.
DHH105 treatment increases PI3-kinase activity and Akt/PKB phosphorylation, contributing to glucose transport. a Isolated rat epididymal adipocytes were treated with 500 μmol/l DHH105 (DHH) for 2 h or 25 ng/ml insulin (INS) for 10 min. CON, untreated control. Cell lysates were prepared and PI3-kinase activity was measured by the conversion of phosphatidylinositol to PIP3, which was visualised by autoradiography after resolution by thin layer chromatography. b The intensity of the PIP3 band was normalised with that of phosphatidylinositol, and the relative amount of PIP3 was calculated by (normalised band intensity of sample)/(normalised band intensity of control). Data are means±SEM of three independent experiments. *p<0.05 as compared with control group. c Adipocytes were treated without or with DHH105 (500 μmol/l) for 4 h in the presence of vehicle (DMSO, white bars), wortmannin (100 nmol/l; grey bars), or LY294002 (50 μmol/l; black bars). Glucose transport was measured by 3H-2-DOG uptake. Data are means±SEM of three independent experiments carried out in triplicate. **p<0.01 compared with the DHH105-treated group in the absence of inhibitors. d Isolated rat epididymal adipocytes were treated with 500 μmol/l DHH105 for 2 h (DHH) or 25 ng/ml insulin for 10 min (INS). CON, untreated control. Cell lysates were prepared and Western blots were performed using anti-phosphorylated Akt/PKB (p-Akt) or anti-Akt/PKB (Akt) antibody. The expression of β-actin was measured as an internal control (β-actin). Results are representative of three independent experiments
Discussion
Many bioactive compounds with anti-diabetic effects have been found in medicinal plants [30–32] including cinnamon [9–13]. In this study, a series of synthesised derivatives of DHH, which was purified from cinnamon, were tested for glucose transport activity in vitro. Among the tested compounds, DHH105 showed the highest glucose transport activity. DHH105 treatment significantly lowered blood glucose levels in type 2 obese (ob/ob) and type 1 non-obese (STZ-treated) diabetic animals as compared with vehicle-treated controls. There were no significant differences in body weight between DHH105-treated and untreated mice and no gross lesions of the examined organs, including liver, kidney, intestine, heart, spleen, and brain, suggesting that there are no apparent side effects of DHH105 treatment (data not shown).
Impaired glucose transport is one of the major factors contributing to insulin resistance in diabetic patients, and type 2 diabetic patients exhibit a marked reduction in insulin-mediated glucose disposal [33, 34]. Thus, we investigated the effect of DHH105 on glucose transport in isolated rat epididymal adipocytes to examine the possible mechanisms involved in the lowering of blood glucose in DHH105-treated diabetic animals. DHH105 increased glucose transport in adipocytes in a dose- and time-dependent manner and significantly increased the maximum speed of transport, but did not change the K m. This indicates that DHH105 significantly increased the number of carriers on the cell surface without changing the affinity of the carrier for its substrate.
GLUT4 is the major glucose transporting molecule in muscle and adipose tissue [4]. In the basal or non-stimulated state, more than 95% of GLUT4 is located in GLUT4 storage vesicles and in the trans-Golgi network of cytoplasmic vesicles close to the plasma membrane [35, 36]. Insulin causes a rapid increase in the amount of GLUT4 at the cell surface in adipocytes and muscle by increasing exocytosis and decreasing endocytosis of GLUT4-containing vesicles [37]. In our study, it appears that DHH105 treatment increases glucose transport by augmenting GLUT4 translocation. DHH105-induced glucose transport was completely inhibited by PAO. PAO has been suggested to inhibit GLUT4-mediated glucose transport by either inhibiting translocation from GLUT4 storage vesicles, by decreasing the efflux of GLUT4 [28], or causing GLUT4 degradation [38]. Whatever the mechanism, it is certain that GLUT4 is critical for DHH105-mediated glucose transport in adipocytes. In addition, we found that the amount of GLUT4 in the plasma membrane fraction was increased after treatment with DHH105, whereas the amount of GLUT4 in the microsomal membrane fractions was decreased at the same time. Taken together, it appears that the translocation of GLUT4 from the microsomal membrane to the plasma membrane is required for DHH105-stimulated glucose transport.
Glucose transport is modulated not only by insulin, but also by other factors such as cellular stress [39, 40]. To confirm the insulin-like actions of DHH105, we determined the effect of DHH105 on glycogen synthesis in adipocytes. DHH105 increased glycogen synthesis to a similar extent as insulin, suggesting that DHH105 may mimic insulin’s effects on glucose metabolism.
Insulin stimulates glucose transport by the translocation of intracellular glucose transporters to the plasma membrane [41, 42]. The binding of insulin to IR-α causes conformational changes in IR-β, which results in the autophosphorylation of IR-β and activation of the insulin receptor tyrosine kinase component of IR-β. Insulin receptor tyrosine kinase phosphorylates IRS1/2 [6], and the phosphorylated IRS-1/2 recruits the regulatory subunit of PI3-kinase (p85), resulting in the activation of the catalytic subunit of PI3-kinase (p110) [5, 43]. Activation of the PI3-kinase signalling pathway generates PIP3-leading to the activation of downstream protein kinases such as Akt/PKB [5, 43]; by contrast, inhibition of PI3-kinase activation blocked GLUT4 translocation [44–46]. Therefore, we examined whether DHH105 affects these signalling pathways. We found that DHH105 treatment phosphorylates IR-β and IRS-1 and activates PI3-kinase, and treatment with a PI3-kinase inhibitor such as LY294002 significantly reduced DHH105-induced glucose transport in adipocytes. These results suggest that DHH105 may bind to the IR and activate the IR signalling pathway, contributing to the enhancement of GLUT4 translocation.
In conclusion, we produced a molecule, naphalenemethyl ester of 3,4-dihydroxycinnamic acid (DHH105), which has an anti-diabetic action. DHH105 activates the insulin signal transduction pathway, subsequently increasing the translocation of GLUT4 and contributing to the increased clearance of peripheral glucose. The blood glucose-lowering effect of DHH105 can be achieved without insulin, and DHH105 does not sensitise insulin action. Thus, DHH105 might have therapeutic potential as an anti-diabetic drug.
Abbreviations
- DHH:
-
dihydroxyhydrocinnamic acids
- DHH105:
-
naphthalenemethyl ester of 3,4-dihydroxyhydrocinnamic acid
- DOG:
-
2-deoxyglucose
- GLUT4:
-
glucose transporter 4 (now known as solute carrier family 2 [facilitated glucose transporter], member 4 [SLC2A4])
- HES:
-
HEPES, EDTA, sucrose
- K m :
-
Michaelis–Menten constant
- KRH:
-
Krebs–Ringer HEPES
- NMR:
-
nuclear magnetic resonance
- PAO:
-
phenylarsine oxide
- PI3-kinase:
-
phosphatidylinositol 3-kinase
- PIP3:
-
phosphatidylinositol 3,4,5-triphosphate
- PKB:
-
protein kinase B
- STZ:
-
streptozotocin
- TBST:
-
Tris-buffered saline with Tween
References
James DE, Piper RC (1994) Insulin resistance, diabetes, and the insulin-regulated trafficking of GLUT-4. J Cell Biol 126:1123–1126
DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J (1985) Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76:149–155
Kruszynska YT, Olefsky JM (1996) Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J Investig Med 44:413–428
Baldwin SA (1993) Mammalian passive glucose transporters: members of an ubiquitous family of active and passive transport proteins. Biochim Biophys Acta 1154:17–49
Whitehead JP, Clark SF, Urso B, James DE (2000) Signalling through the insulin receptor. Curr Opin Cell Biol 12:222–228
Lane MD, Flores-Riveros JR, Hresko RC et al (1990) Insulin-receptor tyrosine kinase and glucose transport. Diabetes Care 13:565–575
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 65:385–411
Bailey CJ (2005) Drugs on the horizon for diabesity. Curr Diab Rep 5:353–359
Anderson RA, Broadhurst CL, Polansky MM et al (2004) Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem 52:65–70
Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA (2003) Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 26:3215–3218
Broadhurst CL, Polansky MM, Anderson RA (2000) Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem 48:849–852
Onderoglu S, Sozer S, Erbil KM, Ortac R, Lermioglu F (1999) The evaluation of long-term effects of cinnamon bark and olive leaf on toxicity induced by streptozotocin administration to rats. J Pharm Pharmacol 51:1305–1312
Imparl-Radosevich J, Deas S, Polansky MM et al (1998) Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signalling. Horm Res 50:177–182
Hoffman WF, Alberts AW, Cragoe EJ Jr et al (1986) 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 2. Structural modification of 7-(substituted aryl)-3,5-dihydroxy-6-heptenoic acids and their lactone derivatives. J Med Chem 29:159–169
Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW (2000) Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature 408:483–488
Olefsky JM (1975) Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest 56:1499–1508
Green A (1986) The insulin-like effect of sodium vanadate on adipocyte glucose transport is mediated at a post-insulin-receptor level. Biochem J 238:663–669
Khil LY, Han SS, Kim SG et al (1999) Effects of brazilin on GLUT4 recruitment in isolated rat epididymal adipocytes. Biochem Pharmacol 58:1705–1712
Berman WF, Bautista JO, Rogers S, Segal S (1976) Metabolism and transport of galactose by rat intestine. Biochim Biophys Acta 455:90–101
Cammalleri C, Germinario RJ (2003) The effects of protease inhibitors on basal and insulin-stimulated lipid metabolism, insulin binding, and signaling. J Lipid Res 44:103–108
Gammeltoft S, Gliemann J (1973) Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta 320:16–32
Ceddia RB, Sweeney G (2004) Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol 555:409–421
Palanivel R, Maida A, Liu Y, Sweeney G (2006) Regulation of insulin signalling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia 49:183–190
Simpson IA, Yver DR, Hissin PJ et al (1983) Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta 763:393–407
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV (1997) Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem 272:30075–30082
Jhun BH, Hah JS, Jung CY (1991) Phenylarsine oxide causes an insulin-dependent, GLUT4-specific degradation in rat adipocytes. J Biol Chem 266:22260–22265
Frost SC, Lane MD, Gibbs EM (1989) Effect of phenylarsine oxide on fluid phase endocytosis: further evidence for activation of the glucose transporter. J Cell Physiol 141:467–474
Lawrence JC Jr, Larner J (1978) Activation of glycogen synthase in rat adipocytes by insulin and glucose involves increased glucose transport and phosphorylation. J Biol Chem 253:2104–2113
de Sousa E, Zanatta L, Seifriz I et al (2004) Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. J Nat Prod 67:829–832
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT (2003) Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod 66:788–792
Carney JR, Krenisky JM, Williamson RT, Luo J (2002) Achyrofuran, a new antihyperglycemic dibenzofuran from the South American medicinal plant Achyrocline satureioides. J Nat Prod 65:203–205
Ginsberg H, Kimmerling G, Olefsky JM, Reaven GM (1975) Demonstration of insulin resistance in untreated adult onset diabetic subjects with fasting hyperglycemia. J Clin Invest 55:454–461
Reaven GM (1983) Insulin resistance in noninsulin-dependent diabetes mellitus. Does it exist and can it be measured? Am J Med 74:3–17
Simpson F, Whitehead JP, James DE (2001) GLUT4—at the cross roads between membrane trafficking and signal transduction. Traffic 2:2–11
Czech MP, Corvera S (1999) Signaling mechanisms that regulate glucose transport. J Biol Chem 274:1865–1868
Millar CA, Shewan A, Hickson GR, James DE, Gould GW (1999) Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes. Mol Biol Cell 10:3675–3688
No authors listed (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853
Gual P, Le Marchand-Brustel Y, Tanti J (2003) Positive and negative regulation of glucose uptake by hyperosmotic stress. Diabetes Metab 29:566–575
Krook A, Wallberg-Henriksson H, Zierath JR (2004) Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc 36:1212–1217
Suzuki K, Kono T (1980) Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA 77:2542–2545
Cushman SW, Wardzala LJ (1980) Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem 255:4758–4762
Shepherd PR, Withers DJ, Siddle K (1998) Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J 333(Pt 3):471–490
Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M (1994) Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem 269:3568–3573
Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR (1994) Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol 14:4902–4911
Sharma PM, Egawa K, Huang Y et al (1998) Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J Biol Chem 273:18528–18537
Acknowledgement
We thank A. Kyle for editorial assistance.
Duality of interest
The authors declare that they have no duality of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
W. Kim and L. Y. Khil contributed equally to the work.
Sadly, J.W. Yoon passed away on 6 April 2006.
Rights and permissions
About this article
Cite this article
Kim, W., Khil, L.Y., Clark, R. et al. Naphthalenemethyl ester derivative of dihydroxyhydrocinnamic acid, a component of cinnamon, increases glucose disposal by enhancing translocation of glucose transporter 4. Diabetologia 49, 2437–2448 (2006). https://doi.org/10.1007/s00125-006-0373-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0373-6