Abstract
Aims/hypothesis
The aim of the study was to investigate the potential of human pancreatic non-endocrine cells to transdifferentiate into endocrine cells that would be capable of secreting insulin in response to glucose and ameliorating insulin-deficient diabetes after transplantation.
Materials and methods
Cell fractions enriched with exocrine cells after human islet isolation were treated with streptozotocin to remove residual beta cells, grown in monolayer culture to allow de-differentiation, transferred to cluster culture for redifferentiation in the presence of activin A, betacellulin, nicotinamide and glucose, supplemented with 10% FCS, and administered to streptozotocin-induced diabetic SCID mice. A subset of cells was transfected with the IPF1 gene (also known as PDX1) before transdifferentiation.
Results
No insulin was detectable in cell preparations after 5 days of treatment with streptozotocin. In monolayer culture, 90% of the streptozotocin-treated pancreatic cells co-expressed cytokeratin-19 and vimentin at 2 weeks and 60% expressed nestin at 4 weeks. Cell cultures with a high proportion of nestin-expressing cells had greater plasticity for transdifferentiation into cells with phenotypic and functional markers of beta cells, this property being significantly enhanced by transfection with IPF1 gene and leading to 15±6.7% insulin-positive cells after transplantation vs. 0.01% of cells transplanted after streptozotocin treatment alone. These cells improved glucose control in all of 42 diabetic mice after transplantation, restoring normoglycaemia in 40%.
Conclusions/interpretation
Human pancreatic cells are a potential source of new glucose-responsive insulin-producing cells that may be developed further for clinical use.
Similar content being viewed by others
Introduction
Pancreatic non-endocrine cells may be a potential source of new beta cells [1–3], as endocrine and exocrine cells in the pancreas share the same developmental origin [4–6]. In culture, endocrine and exocrine cells de-differentiate rapidly and express the same ductal cell marker, CK-19 (human) or CK-20 (rodent) [7–9], indicating that they are de-differentiating in a similar pattern. Furthermore, exocrine and ductal cells of rodents and man have been made to express Pdx1 protein in vitro [10, 11]. Pdx1 is an important transcription factor in pancreatic development and maintenance of the beta cell phenotype [12], and its presence suggests that the exocrine and ductal cells can transdifferentiate into cells with the potential to become endocrine cells and that cells with the ductal phenotype may be an intermediate product between undifferentiated and differentiated cells. Human pancreatic duct cells have been implicated as the origin of new beta cells in systems, without [13, 14] and with [15] the help of ectopic gene expression. Rodent exocrine cells can express insulin [16], supporting the concept that the exocrine cell may be a candidate for new beta cells. However, the goal of generating glucose-responsive beta-cell surrogates in numbers sufficient to reverse diabetes remains elusive and ultimately a human cell source will be required for clinical purposes. In an attempt to enhance the efficacy of beta cell generation from human sources, we first extended the culture of the non-endocrine cells to induce partial de-differentiation. We then introduced redifferentiation in these cells by culturing them in an environment that favoured differentiation of endocrine cells in vitro, with or without prior ectopic expression of IPF1 (also known as PDX1). Finally, we allowed the partially differentiated cells to mature in a diabetic environment in vivo before assessing their ability to correct hyperglycaemia in diabetic mice.
Materials and methods
Reagents
Monoclonal antibody to human insulin (I2018), rabbit anti-human α-amylase antiserum (A8273), glucagon-like peptide-1 (GLP-1) (G3265) and nicotinamide were purchased from Sigma (Sigma-Aldrich, Poole, Dorset, UK). Anti-cytokeratin-19 (Sc-6278) monoclonal antibody and anti-neuroD (Sc-1084) were purchased from Santa Cruz (Santa Cruz Biotechnology, Heidelberg, Germany). Monoclonal antibody against human fibroblast (clone 5B5) and rabbit anti-human Ki-67 antigen antiserum were obtained from Dako (Glostrup, Denmark). Anti-nestin monoclonal antibody (MAB 5326) was purchased from Chemicom International (Temecula, CA, USA). Human-specific vimentin monoclonal antibody (clone V9, Non3005) was purchased from Monosan (Amsterdam, The Netherlands). Recombinant human betacellulin and activin A proteins were from R&D Systems (Oxford, UK).
Culture of human pancreatic non-endocrine cells
Human pancreases were obtained with permission from the local hospital ethical committee and informed consent from families of organ donors (n=16, seven male, nine female; age 18–58 years). Cold ischaemia time was 5.2±1.5 h (mean±SD). The pancreases were subjected to liberase digestion and density gradient fractionation as previously described [17]. Gradient fractions enriched with exocrine cells were collected, washed twice with Hanks’ balanced salt solution and cultured in suspension as clusters [18] in CMRL1066 medium (Invitrogen, Inchinnan, UK), supplemented with 10% FCS and 0.95 mmol/l freshly prepared streptozotocin (STZ; Sigma) at 37°C in a humid 5% carbon dioxide incubator for 5 days, with medium and STZ changed daily to remove contaminating beta cells and dead cells. Cell samples were collected daily for genomic DNA preparation (Promega, Southampton, UK) to determine cell number indirectly, and for analysis of insulin content by immunocytochemical staining, RIA and RT-PCR as previously described [17, 18]. After STZ treatment, the cells were cultured as monolayers. They were seeded at 3×106 cells/10 cm Petri dish and grown initially at approximately 70% confluence in CMRL1066 medium with 55 μmol/l G418 to inhibit the growth of fibroblasts [9] for up to 7 weeks. Cell samples were analysed at intervals of 5–7 days by immunocytochemistry and RT-PCR [18] to assess phenotypic changes. Quantitative PCR (Q-PCR) was used to confirm key RT-PCR experiments and analysed using published methods [19, 20].
Analysis of cell proliferation
Cells from the monolayer cultures were dissociated using trypsin/EDTA and counted at intervals. Cell division was assessed using a 5-bromo-2′-deoxyuridine (BrdU) incorporation assay. For this, cells were seeded 2 h earlier, 65 nmol/l BrdU was added, and culture continued for 16 h. The cells were washed and dissociated and centrifuged to form a pellet. The pellet was cryosectioned and stained for both BrdU and Ki67, an endogenous proliferation marker [21, 22], using published methods [18].
Human IPF1 expression plasmid and transfection
The coding region of the human IPF1 gene was amplified by PCR using a primer set (Table 1). The cDNA was cloned into the expression plasmid pIRES-N1 [23] and partially sequenced. DNA transfections were performed using lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Plasmid carrying the β-galactosidase reporter gene (pCMVβ; BD Biosciences, San Jose, CA, USA) was used to establish optimal transfection conditions, and approximately 30% efficiency was achieved. Briefly, 4×106 cells/10 cm Petri dish were seeded 3 h before transfection. Plasmid DNA (16 μg) was diluted in 1.5 ml of OptiMEM medium (Invitrogen) in tube A and 60 μl of lipofectamine 2000 was diluted with 1.5 ml of OptiMEM medium in tube B. Following incubation at room temperature for 5 min, DNA in tube A was mixed with the lipofectamine 2000 in tube B and incubation was continued at room temperature for another 20 min. Before transfection, the cells were rinsed once with OptiMEM medium and 3 ml DNA/lipofectamine 2000 mixture was added drop-wise onto the cells. The cells were incubated at 37°C in a humid 5% carbon dioxide incubator for 3 h. The transfection mixture was then removed and rinsed once with culture medium, and cultured for another 48 h. The cells were dissociated using trypsin/EDTA and converted into cluster culture [18]. Empty plasmid pIRES-N1 was used as a negative transfection control.
Transdifferentiation induction
To induce the partially de-differentiated non-endocrine cells into low-degree differentiation in vitro, the 4-week monolayer-cultured cells were converted to cluster culture in CMRL1066 overnight [18]. Next morning, the medium was replaced with serum-free CMRL1066 containing 50 nmol/lGLP-1 (Sigma-Aldrich) and incubated for 3 h. Then, a cocktail of differentiation inducers was added at final concentrations of 25 pmol/l activin A, 200 pmol/l betacellulin, 10 mmol/l nicotinamide and 16.5 mmol/l glucose (ABNG; added only in cluster culture), supplemented with 10% FCS. The cells were cultured for additional 4–10 days, with medium and ABNG replaced every 2 days. Cell samples were taken and assessed for expression of beta cell markers.
Semi-quantitative RT-PCR
Total RNA was isolated using an RNA Miniprep Kit (Promega) and quantified using a spectrometer (Gene Quant II; Pharmacia Biotech, Cambridge, UK) at 260 nm wavelength [18]. Briefly, 100 ng of total RNA was converted into first-strand cDNA using reverse transcriptase (Invitrogen) in a volume of 20 μl. The hot-start (Qiagen, Crawley, UK) RT-PCR was used with the following parameters: 95°C for 15 min for one cycle; then 25 cycles of PCR with Ipf1, 28 cycles with NeuroD1 and preproinsulin unless specified otherwise, and 30 cycles with SLC2A2 (also known as GLUT-2) and glucokinase, while β-actin was amplified in parallel for 21 cycles as an internal control using the primers listed in Table 1. The relative quantity of DNA was analysed using a gel program (version 3.0 computer analyser of Media Cybernetics, Silver Spring, MD, USA) and expressed relative to the density of β-actin gene PCR product.
Quantitative PCR
Q-PCR was performed with the LightCycler-2 (Roche Diagnostics, Lewes, UK) using the SYBR Green I fluorescence kit (Roche Diagnostics) and was mainly used to confirm the key semiquantitative RT-PCR. Target genes, such as human preproinsulin (435 bp), NeuroD1 (410 bp), IPF1 (508 bp), SLC2A2 (523 bp) and glucokinase (597 bp), and the reference gene human β-actin (664 bp) were amplified in duplicate and cDNA from at least three donors was tested for each set of experiments. PCR was amplified in 20 μl with 0.25 μmol/l primers and appropriate amounts of DNA template. PCR-grade water was used as a negative control. Amplification efficiency was assessed by using a series of 10× dilutions of plasmid containing the gene or dilutions of cDNA templates. After an initial 15-min preincubation step at 95°C, the following parameters were used: 95°C for 10 s, 53–58°C for 20 s and 72°C for 45 s for 40 cycles. The relative copy number was corrected to 10,000 copies of human β-actin mRNA and expressed as copy number per 10,000 copies of β-actin gene±SD, using the comparative threshold (C t) method [19, 20]: \(2^{{ - \Delta {\text{Ct}}}} \)where ΔC t=(C t of the target gene minus C t of the reference gene). The C t of the genes was determined using thermocycler software (version 3.5; Roche Diagnostics).
Immunochemical staining
The methods used have been described previously [18]. Briefly, cultured cells or tissues were mounted with OCT compound (Merck, Lutterworth, Leics, UK) and cryosectioned at 5 μm intervals. The sections were assessed for the presence of specific antigens by peroxidase-based immunocytochemical staining (Vector Laboratories, Peterborough, UK). The primary antibody was replaced with species-related normal serum to validate specific staining. To visualise the antigens, VIP (violet) substrate (Vector Laboratories) was used. At least 5000 cells per sample were counted.
Animals and surgical procedures
Male SCID mice (20–25 g, C.B-17/Icr), purchased from Charles River Laboratories, Margate, UK, were selected as recipients for transplantation and were maintained in filter cages in the Comparative Biology Centre at King’s College London, according to guidelines of the Home Office (UK) for Animal Scientific Procedures. Diabetes was induced by a single injection of STZ (180 mg/kg i.p.) and confirmed by the presence of hyperglycaemia. The diabetic condition was allowed to stabilise for 7–10 days before transplantation. The diabetic mice were randomly allocated to a transplant recipient group and a sham-operated control group. The surgical procedures have been described [18]. Briefly, under anaesthesia, a median lateral laparotomy was performed and the left kidney was exposed. Approximately 1500 cell clusters (approximately 2.5×106 cells) in a volume of 50 μl were transplanted into the kidney parenchyma tissue by direct insertion of a Hamilton syringe into the kidney at the lower pole, crossing the kidney and reaching the capsule membrane. The cells were discharged into the parenchyma as the syringe was slowly withdrawn. In the sham control group, a cell-free saline injection was made in exactly the same way. Plasma glucose was measured using a G2 blood glucose sensor from MediSense (Abbott Laboratories, Dublin, Ireland).
Intraperitoneal glucose tolerance test
The mice were fasted overnight and the plasma glucose level was determined. A glucose solution at 1.5 g/kg body weight was injected intraperitoneally and plasma glucose levels were determined 30, 60, 90, 120 and 180 min after injection.
At the end of the experiments, blood, kidneys and pancreases were removed. Immunohistochemistry was performed on the tissues for markers of endocrine, exocrine cells, ductal cells, fibroblasts, and vimentin- and Ki67-expressing cells. Cell nuclei were counterstained with propidium iodide (Sigma-Aldrich) and analysed by fluorescence or confocal microscopy. Cells that stained positive and the total number of transplanted cells (total grafted cells) were counted under light fluorescence microscopy and the percentage of positive cells was derived by counting a total of 1500 graft cells per staining. The concentration of human-specific C-peptide was determined by RIA (DSL, Webster, TX, USA). Organs from sham-operated mice were studied in parallel as a negative control.
Data analysis
All data are expressed as mean±SD unless specified otherwise and statistical differences between groups were determined by ANOVA (Microsoft Excel, Office 2000). Differences between groups were considered significant when p<0.05.
Results
Removal of beta cells from human pancreatic non-endocrine cell preparations
Under light microscopy, the exocrine-cell-enriched cell fractions contained <0.3% insulin-positive cells (Fig. 1a) by dithizone staining. Following STZ treatment, beta cells were not detectable by dithizone staining (n=16) or by anti-insulin immunocytochemical staining (n=5, data not shown). The cells were predominantly exocrine cells (79±12% amylase-positive) and ductal cells (15±11% cytokeratin-19-positive, n=16). The expression of preproinsulin mRNA (n=5) was significantly reduced, as determined by semiquantitative RT-PCR (Fig. 1b) following STZ treatment, consistent with the data from the Q-PCR. The number of copies of preproinsulin mRNA/10,000 copies of human β-actin mRNA was reduced from 40 to 3.5 following STZ treatment, a ten-fold decrease (Fig. 1c; n=4, p<0.01). Insulin protein was not detectable by RIA [18] (Fig. 1d; n=5). Cell death occurred during STZ treatment and the cell mass was reduced by 20%, as assessed by the reduction of genomic DNA (Fig. 1e; n=4). In a further confirmation of the removal of beta cells, STZ-treated cells were implanted in the kidneys of the STZ-induced diabetic SCID mice. Fewer than 0.01% of graft cells were subsequently found to be positive for insulin (Fig. 1f, n=4), a 30-fold decrease in beta-cell number, confirming the removal of most beta cells by the isolation procedure and STZ treatment.
Removal of beta cells from exocrine cell preparations. a Typical low-power microscopic image of a routine preparation of human exocrine-cell-enriched cells following dithizone staining, showing the presence of <0.3% of dithizone-positive cells (arrows), with boxed area enlarged (inset). b Semiquantitative RT-PCR showing reduction in expression of preproinsulin in the cells following streptozotocin (STZ) treatment. c. Relative quantitative RT-PCR, confirming the reduction in expression of preproinsulin in exocrine cell preparation following STZ treatment (n=12); **p<0.01. d Reduction of genomic DNA during STZ treatment, indicating that cell loss is more obvious during the initial period of STZ treatment (n=4). e Insulin content in the cells after STZ treatment, showing no difference from the lysate of MCF-7, a cancer cell line that does not express insulin. f Typical microscopic image of a graft of STZ-treated cells 6 weeks after transplantation within the kidney of an SCID mouse. It shows <0.01% cells positive for insulin (violet, arrow; nuclei counterstained with methyl green), which is a 30-fold decrease in beta cells compared with untreated cells (a), indicating that STZ treatment removed most beta cells
Expression of transient markers
During 7 weeks of monolayer culture at ∼70% confluence, there was a rapid decrease in amylase-positive cells. The cell proliferation rate was low until 3 weeks in culture, as determined by the proliferation marker Ki67, BrdU incorporation and cell counting (Fig. 2a). As the initial proliferation rate was low (Fig. 2a), the cell number at day 7 was used as the starting cell number. The cell number decreased to 80% after the first trypsinisation. After the second week in culture, most cells adapted to the culture conditions and appeared healthy, with no microscopic evidence of cell death. Compared with the cell number at 7 days, the fold change in cell number by the end of the second, third and fourth weeks was 0.81±0.03, 1.18±0.03 and 1.45±0.055 (n=4), respectively. The percentage of fibroblast cells in the initial cell preparations was <1% and there was no evidence of rapid proliferation, as there were fewer than 10% fibroblast cells during the 4-week cultures (Fig. 2a). The cells underwent rapid transdifferentiation in vitro. Expression of CK19 increased transiently, with a maximum of 90% of cells expressing CK19 after 2 weeks (Fig. 2a), declining thereafter. The cells expressing CK19 were not the typical duct cells as most of the CK19+ cells also co-expressed vimentin, a marker of mesenchymal cells. Vimentin was not expressed in duct cells in human pancreas tissues (data not shown). Vimentin-expressing cells increased from 3% in the cell preparations to 95% after 2 weeks in monolayer culture (Fig. 2a). The number of CK19-expressing cells decreased rapidly, while vimentin-expressing cells declined relatively slowly and there were still more than 50% of vimentin-expressing cells by 4 weeks (Fig. 2a). Nestin expression (absent from the initial cell preparations) was first detected after 3 days of culture and increased to 60% after 4 weeks of culture, declining thereafter (Fig. 2a). The expression of nestin protein was simultaneous with its mRNA expression (Fig. 2b).
Phenotypic changes in cells cultured in monolayers for up to 49 days. a Phenotype changes during culture. Incorporation of amylase (yellow, n=8); CK19 (dark blue, n=8), nestin (pink, n=8), fibroblasts (green, n=4), vimentin (mid-blue, n=6), Ki67 (light blue, n=5) and BrdU (purple, n=5), assessed by immunocytochemical staining and cell number changes (brown, n=6) by counting. b Expression of nestin mRNA analysed by RT-PCR of cells maintained in monolayer culture
Plasticity of pancreatic non-endocrine cells
Cells in monolayer culture, without a phase of differentiation induction, did not express any mRNA specific for beta cells. We examined the existence of plasticity in the monolayer-cultured cells at various time points by converting them to cluster culture in the presence of differentiation inducers. After cluster culture with the differentiation cocktail ABNG, preproinsulin mRNA was detected in the cells, with maximal expression (Fig. 3a) in the cells following 4 weeks of monolayer culture, coincident with the highest percentage of cells expressing nestin protein (Fig. 2a). These cells also expressed other phenotypic markers of beta cells, including the products of NeuroD1, IPF1, SLC2A2 and glucokinase (Fig. 3b), confirmed by relative quantitative RT-PCR analyses (Fig. 3c, n=4). The best effect was seen after 8 days’ incubation with ABNG. However, insulin protein was not detected. After transplanting these cells into diabetic SCID mice, a small percentage (3±2.3%, n=6) of insulin-producing cells was detected by immunohistochemistry 6 weeks later, but there was no significant reduction in hyperglycaemia in the mice (data not shown).
a RT-PCR showing the expression of preproinsulin and actin mRNA from cells that had been reverted to cluster culture for 8 days in the presence of ABNG differentiation inducers. b Beta cell marker analysis of cells precultured for 28 days in monolayer and subsequently cultured as clusters for 8 days in the absence (−) or presence (+) of differentiation factors of ABNG. c Relative quantitative RT-PCR confirming changes in expression after induction of differentiation
Effect of transfection with IPF1
The cells that had been cultured for 4 weeks were transfected with IPF1 for 48 h and then converted to cluster culture for 8 days in the presence of ABNG as described above. Q-PCR showed a 2.15±0.21- and 3±0.5-fold increase in the expression of preproinsulin and NeuroD1 mRNA, respectively in the IPF1-transfected cells compared with the vector control cells (Fig. 4, n=6). SLC2A2 and glucokinase did not show significant changes (data not shown). Again, despite the increased expression of preproinsulin mRNA, the cells did not contain detectable insulin protein (n=6, data not shown).
RT-PCR analysis of the expression of preproinsulin, IPF1 and NeuroD1 in cells precultured for 28 days in monolayer and subsequently transfected with IPF1 (lanes 1) or the empty vector (lanes 2) after treatment with differentiation factors for 8 days, corrected to the expression of β-actin. The RT-PCR was amplified for 22 cycles in order to distinguish the differential expression level
Effect of transplantation into STZ-induced diabetic mice
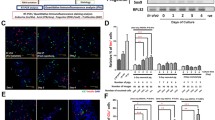
All of the 42 mice that received cells transfected with IPF1 prior to incubation with ABNG showed a progressive reduction in plasma glucose concentrations over a period of 6 weeks (Fig. 5a). Forty per cent of the mice achieved non-fasting blood glucose levels within the non-diabetic range (<9 mmol/l) and all of these showed measurable human C-peptide in their sera, as described below. In contrast, mice transplanted with cells transfected with empty vector remained hyperglycaemic, as did sham-operated mice and mice transplanted with cells isolated from the original STZ-treated pancreatic cells (Fig. 5a). Figure 5b shows the results of intraperitoneal glucose tolerance testing in six of 17 mice that had regained normoglycaemia after implantation with IPF1 transfected cells, together with four sham-operated mice and four normal mice. The active implantation restored glucose tolerance to a near-normal level. The fasted human C-peptide value in mice that had regained normoglycaemia was 0.63±0.31 ng/ml (n=11) and 1.05±0.25 ng/ml (n=6) following the intraperitoneal glucose tolerance test, indicative of graft function, while human-specific C-peptide was undetectable in sham-operated and normal mice. Immunohistochemical analysis of kidney sections of the mice that had returned to normoglycaemia showed healthy grafts that contained 15±6.7% insulin-positive cells (Fig. 5c), 8±2.3% glucagon-positive cells (Fig. 5d) and 3±085% somatostatin-positive cells (Fig. 5e). The grafts of the mice transplanted with vector-transfected cells contained 3±1.85% insulin-positive cells (Fig. 5f; n=6), similar to those without IPF1 transfected cells (data not shown). There were no amylase-positive cells in the grafts, while 4±0.85% fibroblasts, 2.5±0.5% CK19-positive cells and 15.6±3.5% vimentin-positive cells were observed in the grafts. No gross tumour formation was seen in the animals and no tumour-like structures were observed among the grafts. Only 1.5±0.5% (n=8) Ki67+ cells were found in the grafts, suggesting no significant cell proliferation in the grafts. Approximately 50% grafted cells were unidentifiable with the antibodies used. The pancreases of all STZ-treated groups showed numerous sites of damaged islets and only a few residual beta cells (Fig. 5g; n=6) remained, with no significant differences between transplanted and sham-operated groups. There was no evidence of beta-cell proliferation (Ki67-positive cells) in the pancreatic islets (data not shown).
a Non-fasted plasma glucose level over time in SCID mice rendered diabetic with STZ. Values are shown from 10 days before and after transplantation, either with IPF1 (n=42), or with empty vector-transfected-cells (pink, n=12) treated with differentiation factors as in Fig. 3b and Fig. 4, and cells that had received 5 days of STZ treatment (light blue, n=4). Sham-operated mice (yellow, n=8) were used as controls. b. Intraperitoneal glucose tolerance tests on the 6/17 mice that regained normoglycaemia (pink, n=6) with diabetic mice (mid-blue, n=4) and normal mice (dark blue, n=4) as control. c–e The typical immunohistochemical images (green fluorescence) of kidney grafts of STZ-induced diabetic SCID mice 6 weeks after transplantation with IPF1 gene-transfected human pancreatic non-endocrine cells and stained for insulin (c), glucagon (d) or somatostatin (e) in mice that had regained normoglycaemia, in comparison with insulin-expressing cells (green fluorescence) derived from cells that had been transfected with empty vector (f). Insulin-positive cells (c) are boxed and enlarged (inset). g. Typical section of pancreas of STZ-induced diabetic SCID mouse that had regained normoglycaemia, showing residual beta cells (green fluorescence, arrow) in the damaged islets (arrowhead). The nuclei are counterstained with propidium iodide
Discussion
In this study, we explored the potential of human pancreatic non-endocrine cells from normal human pancreas donors as a source of glucose-responsive insulin-producing cells. We developed a three-step protocol: (1) in vitro partial de-differentiation of pancreatic non-endocrine cells; (2) ectopic expression of IPF1 in the partially de-differentiated cells followed by differentiation induction in vitro; and (3) transplantation to allow further differentiation into mature insulin-producing cells in vivo. This protocol produced cells that reversed moderate hyperglycaemia in 40% of diabetic mice and improved glucose control in the remaining 60%. It should be noted that the organ donors covered a broad range of ages and both genders.
The source material for this study was human pancreas donated for islet isolation. To prove the principle, it was important to remove pre-existing beta cells from the cell preparations, thereby ensuring that the insulin-producing cells identified were newly developed. The first step was to take the cell fractions from the pancreatic digests that were depleted of islets. There were no effective ways to remove human beta cells from the cell preparations. Therefore, the cell preparations were treated with STZ, an effective beta-cell toxin in rodents. Human beta cells are relatively resistant to STZ [24]. Nevertheless, STZ has been successfully used in combination with other drugs in treating tumours of beta-cell origin in man [25–27], although beta-cell tumours may react to STZ differently from non-tumour cells. We used a high concentration of STZ (0.95 mmol/l), a concentration that is lethal to mice, in four consecutive treatments. This removed all dithizone-positive cells from our preparations. It remains possible that endocrine cells in the final transplants may have come from undetected residual beta cells, but the numbers make this unlikely. Even after transplantation of the STZ-treated cells, a mere 0.01% of cells were insulin positive and the mice remained hyperglycaemic (Fig. 5a), making it unlikely that the 15.6% insulin-producing cells identified in the fully redifferentiated transplanted cells were carryover beta cells.
Nestin, observed in our de-differentiated cells, is an intermediate filament protein originally described as a neuronal stem cell marker [28]. The association of nestin with pancreatic endocrine cells is not clear [29–32]. The conversion of mouse embryonic stem cells to insulin-secreting cells in vitro involves an intermediate stage when nestin is expressed [33]. It is thought that the expression of nestin may be a marker of a transient cell state intermediate between differentiated and undifferentiated. In addition, we observed that when cell cultures contained a high proportion of nestin-expressing cells, the cells showed a greater capacity to express markers of beta cells following differentiation induction. Therefore, the expression of nestin was taken as a phenotypic marker for transdifferentiation induction in this study.
The molecular mechanisms underlying the transdifferentiation are not clear. We speculate that during culture the non-endocrine cells change their differentiation status and may gain some transient elements, yet to be identified, that allow them to respond better to differentiation induction. The ability to express beta cell markers was closely related to the length of time cells were in monolayer culture, supporting this hypothesis. The transient expression of duct-cell marker is interesting, as cells with duct-cell phenotype have been shown to be capable of expressing markers of beta cells in vitro without [13, 14] or with the ectopic expression of NEUROG3 [15]. More interestingly, the CK19-positive cells in this system were not typical duct cells as they also co-expressed vimentin, a marker of mesenchymal cells, probably connected with epithelial-to-mesenchymal transition [34]. As mention earlier, Pdx-1 is one of the most important transcription factors determining pancreatic development and maintenance of the beta-cell phenotype [12, 35–37]. More importantly, forcing the expression of Ipf1 in liver cells in diabetic mice induces the expression of insulin [38], converting the liver into a pancreas in Xenopus tadpoles [39] and hepatocytes to cells with phenotypes of pancreatic cell in mice [40]. Ipf1 has also been shown to facilitate the expression of insulin in liver cells in mice with damaged islets when their liver cells are undergoing active regeneration [41]. Together with the differentiation inducers, Pdx-1 seems to play a synergistic role in forcing cells with transient plasticity into low-level differentiation towards endocrine cells by functioning as a switch [42], driving a change in the gene expression profile of cells. For example, the transcription factor CEBPB has been used to convert exocrine cell line AR42J into liver cells [43].
The timing of the introduction of IPF1 was also important in our study as the cells from the 28-day monolayer culture showed the greatest ability to express beta cell markers, indicative of cooperation between transient plasticity and the induction of differentiation. The differentiation factors in ABNG [44–46] may also work by altering the gene expression profile. The composition of ABNG was the result of extensive analysis of the redifferentiation of partially de-differentiated human islet cells [18] and the combination was essential in our system. The ABNG cocktail probably provides a balance between survival and stress signals to the cells. A similar phenomenon of environmental factors determining phenotype was observed in rat exocrine cells, as they could express markers of hepatocytes [47] or beta cells [16] under different conditions. Our protocol depended heavily on the in vivo environment, as no insulin protein was detectable until the cells were implanted into the diabetic mice. The hyperglycaemic in vivo environment and close contact with host cells may have contributed to the differentiation of cells into mature beta cells, although the exact mechanism is not yet fully understood. The slow reduction in plasma glucose in the diabetic mice following transplantation suggests the presence of a maturation process before the cells became fully glucose-responsive. Thus, the transdifferentiation of pancreatic non-endocrine cells into endocrine cells required interplay among complex factors, including transient plasticity, the ectopic expression of Ipf1, and the in vitro and in vivo environments. The origin of insulin-producing cells remains uncertain as the rapid phenotypic changes during culture made it difficult to identify the origin of the insulin-producing cells in our system. We have therefore attributed it to the non-endocrine cells as a whole. For reasons discussed above, we do not think the insulin-secreting cells came from a residuum of beta cells in the original cell preparation, although we cannot discount this absolutely.
Ultimately, as shown by the human C-peptide data and the glucose profiles in the intraperitoneal glucose tolerance tests, our protocol resulted in cells that were glucose-responsive, an important criterion for insulin-secreting cells for eventual human use. The culture system we describe is therefore a useful model in which to analyse the underlying molecular mechanisms of the transdifferentiation of human non-endocrine cells to mature, functional endocrine cells, including beta cells, and has the potential to be developed further to generate glucose-responsive insulin-producing cells in large quantity for clinical use.
Abbreviations
- ABNG:
-
activin A, betacellulin, nicotinamide and glucose
- BrdU:
-
5-bromo-2′-deoxyuridine
- GLP-1:
-
glucagon-like peptide-1
- Q-PCR:
-
quantitative polymerase chain reaction
- STZ:
-
streptozotocin
References
Arias AE, Bendayan M (1993) Differentiation of pancreatic acinar cells into duct-like cells in vitro. Lab Invest 69:518–530
De Lisle RC, Logsdon CD (1990) Pancreatic acinar cells in culture: expression of acinar and ductal antigens in a growth-related manner. Eur J Cell Biol 51:64–75
Hall PA, Lemoine NR (1992) Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol 166:97–103
Edlund H (1999) Pancreas: how to get there from the gut? Curr Opin Cell Biol 11:663–668
Edlund H (1998) Transcribing pancreas. Diabetes 47:1817–1823
St-Onge L, Wehr R, Gruss P (1999) Pancreas development and diabetes. Curr Opin Genet 9:295–300
von Schonfeld J, Goebell H, Muller MK (1994) The islet–acinar axis of the pancreas. Int J Pancreatol 16:131–140
Githens S, Schexnayder JA, Moses RL, Denning GM, Smith JJ, Frazier ML (1994) Mouse pancreatic acinar/ductular tissue gives rise to epithelial cultures that are morphologically, biochemically, and functionally indistinguishable from interlobular duct cell cultures. In Vitro Cell Dev Biol Anim 30A:622–635
Kerr-Conte J, Pattou F, Lecomte-Houcke M et al (1996) Ductal cyst formation in collagen-embedded adult human islet preparations. A means to the reproduction of nesidioblastosis in vitro. Diabetes 45:1108–1114
Rooman I, Heremans Y, Heimberg H, Bouwens L (2000) Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia 43:907–914
Gmyr V, Kerr-Conte J, Belaich S et al (2000) Adult human cytokeratin 19-positive cells reexpress insulin promoter factor 1 in vitro: further evidence for pluripotent pancreatic stem cells in humans. Diabetes 49:1671–1680
Miller CP, McGehee RE Jr, Habener JF (1994) IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J 13:1145–1156
Bonner-Weir S, Taneja M, Weir GC et al (2000) In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 97:7999–8004
Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T (2003) Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 52:2007–2015
Heremans Y, Van De Casteele M, in’t Veld P et al (2002) Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol 159:303–312
Lardon J, Huyens N, Rooman I, Bouwens L (2004) Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch 444:61–65
Huang GC, Zhao M, Jones P et al (2004) The development of new density gradient media for purifying human islets and islet quality assessments. Transplantation 77:143–145
Zhao M, Christie MR, Heaton N, George S, Amiel S, Huang GC (2002) Amelioration of streptozotocin-induced diabetes in mice using human islet cells derived from long-term culture in vitro. Transplantation 73:1454–1460
Chakrabarti SK, James JC, Mirmira RG (2002) Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem 277:13286–13293
Ponchel F, Toomes C, Bransfield K et al (2003) Real-time PCR based on SYBR-Green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol 13:18
Hall PA, Richards MA, Gregory WM, d’Ardenne AJ, Lister TA, Stansfeld AG (1988) The prognostic value of Ki67 immunostaining in non-Hodgkin’s lymphoma. J Pathol 154:223–235
Campana D, Janossy G (1988) Proliferation of normal and malignant human immature lymphoid cells. Blood 71:1201–1210
SM Hobbs, (1997) Artificial DNA: bicistronic eukaryotic expression vector pIRES-N1 submitted to EMBL/GenBank/DDBJ databases. AC no. Y11035
Elsner M, Tiedge M, Lenzen S (2003) Mechanism underlying resistance of human pancreatic beta cells against toxicity of streptozotocin and alloxan. Diabetologia 46:1713–1714
Kaltsas GA, Mukherjee JJ, Isidori A et al (2002) Treatment of advanced neuroendocrine tumours using combination chemotherapy with lomustine and 5-fluorouracil. Clin Endocrinol (Oxf) 57:169–183
Oberg K (2003) Diagnosis and treatment of carcinoid tumors. Expert Rev Anticancer Ther 3:863–877
Delaunoit T, Ducreux M, Boige V et al (2004) The doxorubicin–streptozotocin combination for the treatment of advanced well-differentiated pancreatic endocrine carcinoma; a judicious option? Eur J Cancer 40:515–520
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595
Zulewski H, Abraham EJ, Gerlach MJ et al (2001) Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 50:521–533
Selander L, Edlund H (2002) Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev 113:189–192
Piper K, Ball SG, Turnpenny LW, Brickwood S, Wilson DI, Hanley NA (2002) Beta-cell differentiation during human development does not rely on nestin-positive precursors: implications for stem cell-derived replacement therapy. Diabetologia 45:1045–1047
Humphrey RK, Bucay N, Beattie GM et al (2003) Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes 52:2519–2525
Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R (2001) Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292:1389–1394
Kalluri R, Neilson EG (2003) Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784
Jonsson J, Carlsson L, Edlund T, Edlund H (1994) Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371:606–609
Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H (1998) Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 12:1763–1768
Weng J, Macfarlane WM, Lehto M et al (2001) Functional consequences of mutations in the MODY4 gene (IPF1) and coexistence with MODY3 mutations. Diabetologia 44:249–258
Ferber S, Halkin A, Cohen H et al (2000) Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 6:568–572
Horb ME, Shen CN, Tosh D, Slack JM (2003) Experimental conversion of liver to pancreas. Curr Biol 13:105–115
Ber I, Shternhall K, Perl S et al (2003) Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem 278:31950–1957
Koizumi M, Doi R, Toyoda E et al (2004) Hepatic regeneration and enforced PDX-1 expression accelerate transdifferentiation in liver. Surgery 136:449–457
Shen CN, Slack JM, Tosh D (2000) Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol 2:879–887
Tosh D, Shen CN, Slack JM (2002) Conversion of pancreatic cells to hepatocytes. Biochem Soc Trans 30:51–55
Florio P, Luisi S, Marchetti P et al (2000) Activin A stimulates insulin secretion in cultured human pancreatic islets. Endocrinol Invest 23:231–234
Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A (1993) Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest 92:1459–1466
Mashima H, Ohnishi H, Wakabayashi K et al (1996) Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest 97:1647–1654
Lardon J, De Breuck S, Rooman I et al (2004) Plasticity in the adult rat pancreas: transdifferentiation of exocrine to hepatocyte-like cells in primary culture. Hepatology 39:1499–1507
Acknowledgements
We thank Ms H. Mandefield, the transplantation coordinators of South-Thames and King’s College Hospital, and relatives of the organ donors for human pancreases. We thank Professors A. McGregor, King’s College GKT Medical School, King’s College London and J. Price, Institute of Psychiatry, King’s College London, for critical reading of the manuscript and their invaluable comments. We thank Dr J. Jones for insulin and human C-peptide analysis, and Dr M. Arno, at the Genomics Centre, School of Life Sciences, King’s College London, for his assistance in the real-time PCR experiments. This work was supported by a research grant from Dixons Charitable Trust, UK, and the King’s College Hospital Charitable Trust.
Duality of Interest
The authors declare that they have no financial interest in this study. The funding bodies do not have a role in collecting, analysing or publishing the data in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, M., Amiel, S.A., Christie, M.R. et al. Insulin-producing cells derived from human pancreatic non-endocrine cell cultures reverse streptozotocin-induced hyperglycaemia in mice. Diabetologia 48, 2051–2061 (2005). https://doi.org/10.1007/s00125-005-1888-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1888-y