Abstract
The STOP-NIDDM Trial has shown that acarbose treatment in subjects with impaired glucose tolerance is associated with a significant risk reduction in the development of diabetes, hypertension and cardiovascular complications. Kaiser and Sawicki have accused the investigators of the STOP-NIDDM Trial of major biases in the conduct of the study, of manipulating the data and of conflict of interest. The aim of this paper is to present data and explanations refuting these allegations.
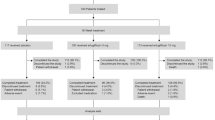
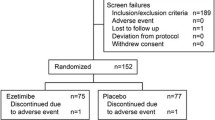
In the STOP-NIDDM Trial, 61 subjects were excluded from the efficacy analysis before unblinding for legitimate reasons: failure to satisfy major entry criteria (n=17) and lack of post-randomisation data (n=44). Blinding and randomisation were carried out by an independent biostatistician. Titration of placebo/acarbose is well described in the protocol and in the study design paper.
Of the study population, 9.3% had a fasting plasma glucose of ≥7.0 mmol/l at screening and could have been diabetic according to the new diagnostic criteria. However, even if these subjects are excluded, patients having acarbose treatment still saw a significant risk reduction in the development of diabetes (p=0.0027). The changes in weight are consistent in different publications and are related to different times of follow-up and assessment. Weight change does have an effect on the development of diabetes, but acarbose treatment is still effective even after adjusting for this (p=0.0063). The cardiovascular endpoints were a clearly designated assessment in the original protocol, and only those defined in the protocol and ascertained by the independent Cardiovascular Event Adjudication Committee were used in the analysis. Hypertension was defined according to the most recent diagnostic criteria.
The STOP-NIDDM Trial results are scientifically sound and credible. The investigators stand strongly behind these results demonstrating that acarbose treatment is associated with a delay in the development of diabetes, hypertension and cardiovascular complications in a high-risk population with IGT.
Similar content being viewed by others
Introduction
The STOP-NIDDM Trial has presented data indicating that treatment of subjects with IGT with the α-glucosidase inhibitor, acarbose, is associated with a 36% reduction in the risk of progression to diabetes based on two consecutive OGTTs [1]. Furthermore, acarbose treatment was also shown to be associated with a 34% risk reduction in the development of new cases of hypertension and a 49% risk reduction in cardiovascular events in subjects with IGT [2]. The rationale for the use of acarbose was based on our understanding of the pathophysiology of Type 2 diabetes [3] and on a preliminary study showing that treatment of IGT subjects with the drug is associated with a significant reduction in insulin resistance as well as a decrease in postprandial hyperglycaemia and hyperinsulinaemia [4]. The use of acarbose was also supported by the observation that postprandial hyperglycaemia is a strong independent predictor for the development of Type 2 diabetes and cardiovascular disease [5, 6, 7].
Kaiser and Sawicki have recently published a paper in Diabetologia [8] in which they accuse the investigators of the STOP-NIDDM Trial of major biases in the conduct of the study, of manipulating the data and of conflict of interest. Here, the investigators would like to respond to the issues raised by Kaiser and Sawicki and to show that their criticisms are unfounded and flawed by misinterpretations, biases and inappropriate valued judgements.
Methods used in the STOP-NIDDM Trial
“Modified” intent-to-treat population
Subjects were enrolled into the study if they had IGT based on a 75-g OGTT and a fasting plasma glucose (FPG) of ≥5.6 mmol/l and <7.8 mmol/l [9]. Altogether, 1429 subjects were randomised into the study. As the data were obtained, quality control was applied and any incomplete or inadequate data were faxed back to the site for completion or with queries. As it happened, 17 subjects did not have IGT based on their first OGTT: 13 were diabetic and four were normal. Furthermore, no post-randomisation glucose data could be collected for 44 subjects: 14 of these withdrew their consent and the others failed to attend subsequent visits. After discussion with the Data Safety and Quality Review Committee (chaired by C. Clark, Indiana University School of Medicine, Indianapolis, Ind., USA), the Steering Committee decided to exclude these 61 subjects from the efficacy analysis while everybody was still blinded to treatment (the investigators, the patients and Bayer, the sponsor). This procedure is legitimate and is in accordance with the ICH-E9 guidelines “Notes for guidance on statistical principles for clinical trials” [10]. Our reasons for excluding the 61 subjects are explicitly mentioned in these guidelines: “The failure to satisfy major entry criteria” (the 17 subjects excluded because they did not have IGT) and “lack of any data post-randomisation” (the 44 subjects excluded because they did not have any efficacy post-randomisation data). In light of this, the term “modified” to describe our intent-to-treat population was not strictly necessary. Nevertheless, the reviewers and editorial staff of JAMA, following their rigorous reviews, insisted that we used the term. Moreover, the term “drop-outs” used by Kaiser and Sawicki is misleading and inappropriate. This term usually refers to patients involved in a study who terminate their participation prematurely. No subject in our study was excluded from the (modified) intent-to-treat analysis for that reason. The characteristics of the 61 excluded subjects are shown in Table 1 and are compared with those of the so-called “modified intent-to-treat population”. Most of the numbers are different from those given by Kaiser and Sawicki, and it is not obvious to us how they arrived at their values.
Randomisation
Randomisation was carried out by W. Taylor from McMaster University (Hamilton, Ont, Canada), a member of the Data Safety and Quality Review Committee, and is well described in both the Lancet and the JAMA papers [1, 2]: “Randomisation was done using a computer program allocation sequence that was stratified by centre. Randomisation was done in blocks of 4 and 6 to minimise the chance that the investigators could guess the treatment assignment. Numbered drug containers were used to implement the random allocation process. Since the random code was stratified by centre, the patients were randomised sequentially at each centre”. The term “sequentially” means “arranged in sequence, in order of succession; consecutive or following in regular order without a break” (Webster dictionary). In other words, the numbered containers were assigned to each enrolled subject in consecutive order. In contrast to what is suggested by Kaiser and Sawicki, the investigators did not have any role in the numbering of the containers, and it is not possible that “allocation concealment could have been influenced”. Since blinding was carried out by an independent biostatistician, the sponsor of the study (Bayer) was also blinded to treatment. Insinuating that they manipulated the data is a major unfounded accusation, which is surprising, unworthy and unexpected from any credible scientific investigator.
Titration of the study medication
The study protocol states: “At randomisation (visit 2), subjects will take either placebo or active drug 50 mg OD for the first 2 weeks, placebo or active drug 50 mg BID for the next 2 weeks and placebo or active drug 50 mg TID until visit 3. At visit 3, subjects will be uptitrated to placebo or active drug 100 mg TID for the rest of the study. By using titration, subjects are expected to increase their tolerance to the study drug. If a subject cannot tolerate the 100 mg TID dose and severe gastrointestinal adverse events are developing, he/she may take 50 mg TID dose for 1 week or 2 weeks, and then retry the 100 mg TID dose. If the subject still cannot tolerate the high dose, the subject will be maintained on the highest tolerable dose for the rest of the study”. Therefore, in contrast to what is suggested by Kaiser and Sawicki, both placebo and acarbose (identical in shape, size and colour) were titrated. This is well described in the original paper where the study design was detailed: “To avoid or minimise the gastrointestinal side effects of acarbose, its administration was started at 50 mg/day and titrated gradually (50 mg/day per 2 weeks) to a maximum of 100 mg TID or the maximum tolerated dosage” [11]. Since this was a double-blind, placebo-controlled study, it is clear that the placebo was explicitly included. This was referred to in the Lancet paper as well: “...the drug was started at 50 mg per day, and increased gradually to a maximum of 100 mg 3 times daily or to the maximum tolerated dose” [1]. In the JAMA paper, we refer to the original paper [11] and state that the “eligible patients were randomised to receive placebo or acarbose 100 mg TID, taken with the first bite of each meal” [2]. As opposed to Kaiser and Sawicki, we fail to see any contradiction in any of these official publications where dose titration is consistent with the protocol.
The Steering Committee
Again, Kaiser and Sawicki are making an inappropriate valued judgement when they raise the issue of conflict of interest due to the fact that some Bayer employees were members of the Steering Committee. The committee was composed of the principal investigator (J.-L. Chiasson) and representatives of the five regions involved in this international study: R. G. Josse from Canada, M. Hanefeld from Germany, M. Laakso from Scandinavia, A. Karasik from Israel and R. Gomis from Spain. Since the infrastructure for monitoring and data collection was provided by Bayer, it was normal to have a representative from the company in each region. Throughout the course of the study, the Bayer representatives changed. Since the research budget had to be adjusted over time, it was necessary to have some Bayer people around the table. However, all of these people were non-voting members, and all decisions and amendments were decided upon by the investigators only. Furthermore, it is common practice to have company employees on the Steering Committee of major clinical trials. The STOP-NIDDM Trial was initiated by the investigators. In fact, the preliminary study published in Diabetes Care in 1996 [4] was done to get on the Diabetes Prevention Program, since we knew that the National Institutes of Health would be calling for sites. When they did, however, they decided that they would not include any site from outside the USA [12]. Two of the Canadian investigators (J.-L. Chiasson and R. G. Josse) then went to Germany to propose the project to Bayer. It was decided on the spot that they would provide an unrestricted research grant for the STOP-NIDDM Trial. As such, the STOP-NIDDM Trial was an investigator-initiated project and remained under the strict control of the investigators throughout the duration of the study. Furthermore, the data analysis, the statistical analysis and the writing of the manuscripts were the sole responsibility of the investigators. Bayer played no role in these aspects of the study. The interim analyses were carried out by W. Taylor from McMaster University, a member of the Data Safety and Quality Review Committee. The final statistical analysis was contracted out to an independent biostatistician (G. Saunders Enterprises, Drayton Valley, Alta, Canada).
Results and discussion of the STOP-NIDDM Trial
Fasting plasma glucose of ≥7.0 mmol/l
In 1995, when the study was initiated, the cut-off point for the diagnosis of diabetes based on FPG was ≥7.8 mmol/l. The subjects could therefore be enrolled into the study if they had IGT and an FPG of ≥5.6 and <7.8 mmol/l. The new diagnostic criteria decreased the FPG cut-off point to 7.0 mmol/l [13]. As it happened, 9.3% of subjects had an FPG of ≥7.0 mmol/l at screening, 74 (11%) in the placebo group and 53 (8%) in the acarbose group. Firstly, it must be emphasised that one FPG measurement of ≥7.0 mmol/l is not sufficient for a diagnosis of diabetes. This has to be confirmed on a separate day by a second FPG of ≥7.0 mmol/l, according to standard diagnostic criteria [13]. Secondly, even if we exclude the 127 patients with an FPG of ≥7.0 mmol/l (which is not consistent with the study protocol), the effect of acarbose on the conversion of IGT to diabetes still remains significant (hazard ratio = 0.75 [95% CI: 0.62–0.90]; p=0.0027). Therefore, the effect of acarbose on the conversion of IGT to diabetes cannot be attributed to the difference in the number of subjects with an FPG of ≥7.0 mmol/l at screening in the two groups as suggested by Kaiser and Sawicki.
Change in body weight and incidence of diabetes
Kaiser and Sawicki suggest that we report different weight change in different publications (0.5 kg in the Lancet paper [1] versus 1.15 kg in the JAMA paper [2]). Both numbers are correct. In the Lancet paper, we report the mean weight change over the whole study period which varied from 3 to 5 years depending on the patient. In the JAMA publication, the mean change over 3 years is reported. This suggests that some of the patients started gaining weight after 3 years and others stopped gaining weight after 3 years. Cox proportional hazard analysis showed that weight loss was associated with a decreased risk of diabetes (p<0.0001). This is mentioned in the Lancet paper [1]. However, even after adjusting for weight change, the effect of acarbose in reducing the risk of diabetes was still significant (p=0.0063). The nutritional evaluation data based on 3-day nutritional diaries have not yet been analysed. However, similar nutritional evaluations carried out in diabetic subjects on acarbose did not show any change in dietary habit in patients on acarbose [14, 15].
Cardiovascular events
In contrast to what is insinuated by Kaiser and Sawicki, the cardiovascular endpoints were all listed and defined in the original protocol dated March 14 1995, page 47, item 10.1.3:
2. Cardiovascular events
The following cardiovascular events will be documented:
-
cardiovascular death
-
myocardial infarction
-
stroke or cerebrovascular accidents
-
angina
-
congestive heart failure
-
peripheral vascular disease
-
revascularization procedure
As such, the cardiovascular endpoints in the JAMA paper were all clearly listed and defined [2]. Furthermore, a “Cardiovascular Event Adjudicating Committee” composed of three independent cardiologists blinded to treatment ascertained all the cardiovascular events reported in the JAMA paper. Before evaluating the events, they produced a “Cardiovascular Event Adjudicating Manual” further detailing the diagnostic criteria for each of the cardiovascular endpoints listed in the protocol. The blinded committee retained 47 subjects in whom one or more cardiovascular events were confirmed according to these rigid criteria. Of these 47 subjects, 32 were in the placebo group and 15 were in the acarbose group (p=0.033) [2]. Kaiser and Sawicki confuse the cardiovascular endpoints that were predefined in the protocol with the adverse events reported by the investigators. These included arrhythmia, thrombophlebitis, ECG abnormalities and syncope, which were not retained by the Cardiovascular Event Adjudicating Committee. Only the events predefined as cardiovascular endpoints in the original protocol and confirmed according to pre-specified criteria by the independent and blinded members of the committee were retained for analysis.
Selective non-follow-up
Kaiser and Sawicki correctly point out that for patients with risk of cardiovascular events, the number of patients at risk with acarbose treatment is smaller than the number of patients at risk in the placebo group. This is due to the fact that premature discontinuation of study medication was more frequent in the acarbose group. Subjects who discontinued the study medication prematurely and had no cardiovascular event were not included beyond the time they stopped the study medication. Only those who had a cardiovascular event were included in the analysis. However, even the inclusion of the subjects discontinuing the study medication prematurely and not having any cardiovascular event does not change the statistical significance (hazard ratio = 0.49 [95% CI: 0.27–0.91]; p=0.025, or p=0.018 by log rank test]. We acknowledge that although this is a clear determination, it is a secondary objective in the original protocol and that the number of subjects with events is relatively small (n=47). Thus, these interesting observations can only be regarded as capable of generating hypotheses. However, there are other findings that support the observed protective effect of acarbose on cardiovascular complications. In a subgroup of patients (n=115), the carotid intima media thickness was measured before randomisation and at the end of the study [16]. Acarbose treatment was associated with a significant decrease in the progression of intima media thickness, an accepted surrogate for atherosclerosis (p=0.027) [16]. Furthermore, in a recent meta-analysis of Type 2 diabetic patients [17], acarbose treatment was associated with a significant reduction in cardiovascular events, even after adjusting for other risk factors (p=0.006).
Hypertension
The diagnostic criterion for hypertension has been a moving target over the last 15 years. While it used to be ≥160/90 in the early 1990s [18], it was decreased to >140/90 in the late 1990s [19] and further decreased to ≥140/90 more recently [20]. In the Lancet paper, the diagnosis of hypertension was based on a blood pressure of >140/90; with this criterion, 629 subjects (46%) had hypertension [1]. In the JAMA paper, we used the most recent criterion of ≥140/90; this gave a total number of 702 subjects (51%) at baseline [2]. Kaiser and Sawicki are correct in that all subjects were included in the analysis. However, even if we exclude the subjects with hypertension at baseline, the effect of acarbose on the development of new cases of hypertension is still significant (hazard ratio = 0.59 [95% CI: 0.39–0.91]; p=0.016).
Whether hypertension had been diagnosed by the family physician was not documented. However, we know that most of the subjects were diagnosed by the investigators, since blood pressure was measured at the 3-monthly visits. We would not like to question the ability of the family physicians to diagnose and treat hypertension. Remember, they were the first line physicians in the United Kingdom Prospective Diabetes Study [21].
Missing data
We did not look at the postprandial plasma glucose. That would have required a mixed meal test. Kaiser and Sawicki state “that such an evaluation had been planned”, quoting the study design paper [11]. That paper states: “Among the secondary objectives is the assessment of any improvement in glucose tolerance (i.e. reversal of IGT to NGT, based on an OGTT) that could result from acarbose treatment”. No meal test was planned. Though the postprandial plasma glucose was not measured, the 2-hour plasma glucose after 75 g glucose was measured, and the analysis of this information is reported in the JAMA paper in Tables 2 and 3 [2]. The 2-hour plasma glucose at baseline was not related to the development of cardiovascular disease or to the development of hypertension. Nevertheless, the 2-hour plasma glucose after 75 g glucose did decrease significantly under acarbose treatment (p<0.0001, data not yet published). However, the change in 2-hour plasma glucose after 75 g glucose is not a good surrogate for the effect of acarbose on postprandial plasma glucose, since the drug itself does not affect the monosaccharide glucose absorption. Therefore, it does not reflect the effect of the drug on 2-hour plasma glucose after a mixed meal containing polysaccharides.
Furthermore, the relationship between baseline HbA1c and the development of cardiovascular events and hypertension is also mentioned in the JAMA paper [2]. Again, this relationship was not significant and thus the HbA1c data were not described in more detail.
The conflicting data on the number of subjects excluded because they did not have any post-randomisation data
Kaiser and Sawicki correctly pointed out that there was a typographical error in the Lancet paper, where it was stated that there were 20 subjects in the placebo group and 24 in the acarbose group who did not have post-randomisation data [1]. This was corrected in the JAMA paper to 21 in the placebo group and 23 in the acarbose group for a total of 44 [2]. As mentioned above, these were excluded for legitimate reasons before unblinding the treatment assignment. As such, the typographical error had no impact on the number of subjects who did not have IGT, as suggested by Kaiser and Sawicki.
The 3-month washout period
The washout period has to be interpreted with caution. Excluded from the washout period were those who discontinued the study medication prematurely and those who had converted to diabetes. Therefore, the analysis is done on a selected subgroup of the “modified” intent-to-treat population. As reported in the Lancet paper, the incidence of diabetes in the washout period was higher in the group originally assigned to acarbose than in the group first randomised to placebo [1]. The most that can be concluded from this observation is that acarbose treatment should probably be continued to maintain the preventive effect of the drug on the development of diabetes.
This is not different for metformin or troglitazone in the Diabetes Prevention Program Study [22, 23]. The subjects assigned to metformin/placebo had an OGTT 1–2 weeks after discontinuing the drug; 8% of those originally on metformin converted to diabetes compared with 5.2% in the placebo group. After discontinuing troglitazone, the incidence of diabetes increased to that of the placebo group. The Troglitazone in Prevention of Diabetes Study suggested that troglitazone could have a prolonged effect on the incidence of diabetes in women with a history of gestational diabetes [24], but this was based on only seven events over 8 months. Overall, the data support the notion that to maintain the beneficial effect on the reduction in the incidence of diabetes in a high-risk population, any intervention has to be continued. However, in the STOP-NIDDM Trial, even if we include the washout period in the Cox proportional hazard model, the result still remains statistically significant (hazard ratio = 0.85 [95% CI: 0.72–1.0]; p=0.0498). Having said this, this additional analysis is not in accordance with the study protocol and cannot replace the primary analysis.
Follow-up duration
It is correct that the follow-up duration was slightly longer in the placebo group compared with that in the acarbose group. This does not affect the Cox proportional hazard analysis which takes into consideration the differences in follow-up time.
Inadequate blinding
Blinding is always a concern when studying drugs that have specific side effects, such as metformin, angiotensin-converting enzyme inhibitors, or acarbose. Furthermore, the potential side effects must be explained to the patients before they sign the consent form.
That was why we started acarbose/placebo at a low dose and titrated gradually to minimise the gastrointestinal side effects as shown by May [25]. Furthermore, as part of the protocol, all patients and all investigators were asked to guess the treatment assignment at the end of the study. This is presented and discussed in the JAMA paper [2]: “Forty-eight percent of patients receiving placebo and 79% receiving acarbose thought they were taking the active drug. Physicians guessed use of acarbose correctly in 69% and incorrectly in 31% of the cases and guessed use of placebo correctly in 64% and incorrectly in 36% of the cases”. Therefore, for both patients and physicians, approximately two-thirds guessed correctly and one-third guessed incorrectly. By chance alone, one would expect 50% to guess correctly. Since the most common side effect, flatulence, is a common physiological occurrence, one would expect more than 50% to guess acarbose. This is exactly the result we had. We doubt very much that this could explain the 50% risk reduction in cardiovascular events. Nobody is questioning the validity of the lifestyle modification studies in which everybody was unblinded [12, 26].
Prevention versus delaying or “masking”
The question as to whether we are preventing, delaying or simply masking Type 2 diabetes is a valid one. In fact, the same question can be asked for metformin, troglitazone and lifestyle modification. However, unlike metformin and troglitazone, acarbose is not absorbed, and as such should not have any pharmacological effect beyond its transit in the gut. All these interventions have one thing in common: they decrease insulin resistance [4, 27, 28, 29]. This effect of acarbose is obviously an indirect one. The only known direct effect of acarbose is the slowing of the hydrolysis of disaccharides and polysaccharides, thereby delaying absorption of glucose by the gut which decreases postprandial plasma glucose. It does not, however, have any effect on the absorption of the monosaccharide glucose during the OGTT [30] or on the disposition of the absorbed glucose, and therefore cannot mask diabetes. In the STOP-NIDDM Trial, not only was there a decrease in the incidence of diabetes, but there was also an increase in the reversion of IGT to NGT [1]. These are significant endpoints that are definitely occurring under the “mask” proposed by Kaiser and Sawicki. In addition, in a similar way to with metformin and lifestyle modification, it is not unexpected that if acarbose is discontinued, the beneficial protective effect of the intervention wears off over time. We believe it is reasonable to say that, in general, most prevention studies, particularly for diabetes and cardiovascular disease, are probably only delaying the outcome.
Conclusion
We can conclude that there is no contradiction and no conflict in the data from the STOP-NIDDM Trial as reported in peer-review journals. This is true for the study design paper published in Diabetes Care [11], the Lancet paper [1] and the JAMA paper [2]. We fully understand and endorse the need for critical assessment and appraisal of published data. We are somewhat surprised that Kaiser and Sawicki failed to appreciate that our papers were rigorously and extensively reviewed by external reviewers and editorial staff from high-impact journals. As such, the STOP-NIDDM Trial data are scientifically sound and credible. They demonstrate that acarbose treatment is associated with a 36% risk reduction in the development of diabetes in high-risk subjects with IGT. They also very strongly suggest that acarbose, with its postprandial mode of action, is associated with a risk reduction in the development of cardiovascular disease and hypertension.
Abbreviations
- BID:
-
two times a day
- FPG:
-
fasting plasma glucose
- OD:
-
once a day
- STOP-NIDDM:
-
Study to Prevent Non-Insulin-Dependent Diabetes Mellitus
- TID:
-
three times a day
References
Chiasson JL, Josse RG, Gomis R et al. (2002) Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359:2072–2077
Chiasson JL, Josse RG, Gomis R et al. (2003) Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. The STOP-NIDDM Trial. JAMA 290:486–494
DeFronzo RA (1988) Lilly Lecture. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687
Chiasson J-L, Josse RG, Leiter LA et al. (1996) The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care 19:1190–1193
Heine RJ, Nijpels G, Mooy JM (1996) New data on the rate of progression of impaired glucose tolerance to NIDDM and predicting factors. Diabetic Med 13 [Suppl 2]:S12–S14
Bonora E, Muggeo M (2001) Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia 44:2107–2114
Qiao Q, Tuomilehto J, Borch-Johnsen K (2003) Post-challenge hyperglycaemia is associated with premature death and macrovascular complications. Diabetologia 46 [Suppl 1]:M17–M21
Kaiser T, Sawicki PT (2004) Acarbose for prevention of diabetes, hypertension and cardiovascular events? A critical analysis of the STOP-NIDDM data. Diabetologia 47:575–580
National Diabetes Data Group (1979) Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 28:1039–1057
No authors listed (1999) ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med 18:1905–1942
Chiasson J-L, Gomis R, Hanefeld M et al. (1998) The STOP-NIDDM Trial. An international study on the efficacy of an α-glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: rationale, design, and preliminary screening data. Diabetes Care 21:1720–1725
Knowler WC, Barrett-Connor E, Fowler SE et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Gavin JR III, Alberti KGMM, Davidson MB et al. (1997) Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197
Chiasson J-L, Josse RG, Hunt JA et al. (1994) The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med 121:928–935
Lindstrom J, Tuomilehto J, Spengler M (2000) Acarbose treatment does not change the habitual diet of patients with Type 2 diabetes mellitus. The Finnish Acargbos Study Group. Diabet Med 17:20–25
Hanefeld M, Chiasson JL, Koehler C et al. (2004) Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke 35:1073–1078
Hanefeld M, Cagatay M, Petrowitsch T et al. (2004) Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 25:10–16
Carruthers SG, Larochelle P, Haynes RB et al. (1993) Report of the Canadian Hypertension Society Consensus Conference: 1. Introduction. Can Med Assoc J 149:289–293
Feldman RD, Campbell NR, Larochelle P (1999) Clinical problem solving based on the 1999 Canadian recommendations for the management of hypertension. CMAJ 161 [Suppl 12]:S18–S22
Practice Guidelines Writing Committee (2003) Practice guidelines for primary care physicians: 2003 ESH/ESC Hypertension Guidelines. J Hypertens 21:1779–1786
No authors listed (1993) Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens 11:309–317
The Diabetes Prevention Program Research Group (2003) Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 26:977–980
The Diabetes Prevention Program Research Group (2003) Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program (DPP). Diabetes 52 [Suppl 1]:A58
Buchanan TA, Xiang AH, Peters RK et al. (2002) Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 51:2796–2803
May C (1995) Efficacy and tolerability of stepwise increasing dosage of acarbose in patients with non-insulin-dependent diabetes (NIDDM), treated with sulphonylureas. Diabetes Und Stoffwechsel 4:3–8
Tuomilehto J, Lindstrom J, Eriksson JG et al. (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
Frias JP, Yu JG, Kruszynska YT et al. (2000) Metabolic effects of troglitazone therapy in type 2 diabetic, obese, and lean normal subjects. Diabetes Care 23:64–69
Dela F, Mikines KJ, Von Linstow M et al. (1992) Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol 263:E1134–E1143
Goodpaster BH, Kelley DE, Wing RR et al. (1999) Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48:839–847
Jenkins DJA, Taylor RH, Goff DV et al. (1981) Scope and specificity of acarbose in slowing carbohydrate absorption in man. Diabetes 30:951–954
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Chiasson, JL., Josse, R.G., Gomis, R. et al. Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia 47, 969–975 (2004). https://doi.org/10.1007/s00125-004-1409-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1409-4